VZV infection diagnosis and detection kit based on chemiluminescence immunoassay
A chemiluminescence immunization and detection kit technology, applied in the fields of medicine and clinical biology, can solve the problems affecting the effectiveness of PCR results, such as vaccines, and achieve the effect of reducing false negatives and false positives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 sample selection and processing
[0037] 1. Sample selection
[0038] For the purposes of the present invention, two types of samples are required, including experimental group samples and control samples. Samples in the experimental group refer to samples from patients suspected of being infected with VZV, preferably patients with obvious symptoms of VZV infection, more preferably samples from patients with confirmed positive diagnostic results based on ELISA or PCR analysis. Control samples refer to samples from patients not infected with VZV, preferably healthy people who have not been vaccinated against any VZV vaccine. Sampling requires the consent of each patient and should be conducted according to ethical practice.
[0039] 2. Sample Processing
[0040] The present invention is performed using human clinical samples, preferably human blood samples, and has been approved by an ethics committee. Blood samples were obtained from forearm punctures of p...
Embodiment 2
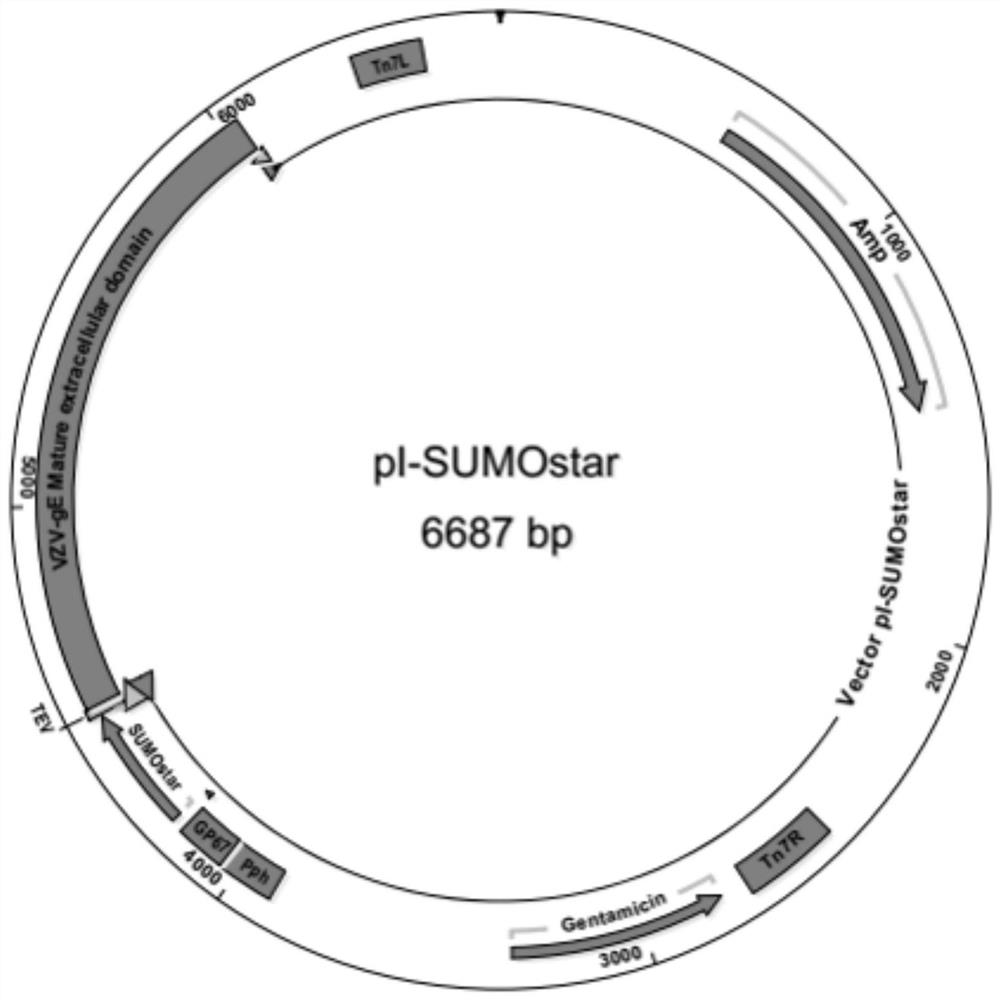
[0041] Example 2 The construction of the baculovirus expression system expressing recombinant His-SUMOstar-VZV-gE protein
[0042] 1. Construction of a recombinant transformation vector carrying the VZV-gE sequence
[0043] To achieve the purpose of the present invention, obtain the gene sequence of the mature extracellular region of VZV-gE encoding VZV, use the following forward and reverse primers to obtain the VZV-gE extracellular structure of accession number MH709377.1 (SEQ ID NO:1) Domains; 5'-ATTTCCAAGGTTCTTCCGTCTTGCGATACGATGATTTTCACATC-3' (SEQ ID NO: 2) and 5'-GACAAGCTTGGTACTTAATATCGTAGAAGTGGTGACGTTCCGGG-3' (SEQ ID NO: 3). Meanwhile, the histidine tag-modified transformation vector pI-SUMOsec-Star-His was linearized by the following primers (forward: 5'CTTCTACGATATTAAGTACCAAGCTTGTCGAGAAGTACTAGAGG3' (SEQ ID NO: 4) and reverse: 5' TATCGCAAGACGGAAGAACCTTGGAAATAAAGATTCTCGCTGCC3' (SEQ ID NO: 5)), the primers comprise sequences overlapping the 5' and 3' end sequences of VZV...
Embodiment 3
[0048] Expression and purification of embodiment 3VZV-gE protein
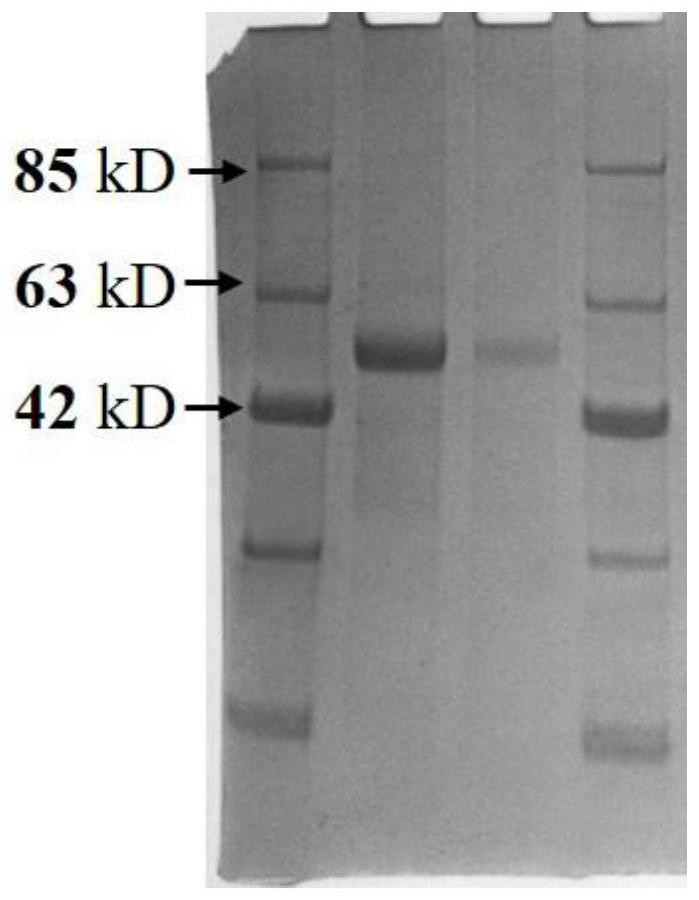
[0049] 1. Expression of recombinant His-SUMOstar-VZV-gE protein
[0050] When in optimal expression conditions, infect 2×10 6 Spodoptera frugiperda (Trichoplusia ni) (High Five, Hi5) cells and incubated at 27°C in a spin shaker. The medium does not contain serum, only penicillin and streptomycin (PS) as antibiotics. After about 3 to 4 days, the cell culture was stopped, and the expressed recombinant protein was collected by centrifuging the culture (30 minutes at high speed) (rotating speed: 5000 g), so as to perform subsequent protein purification.
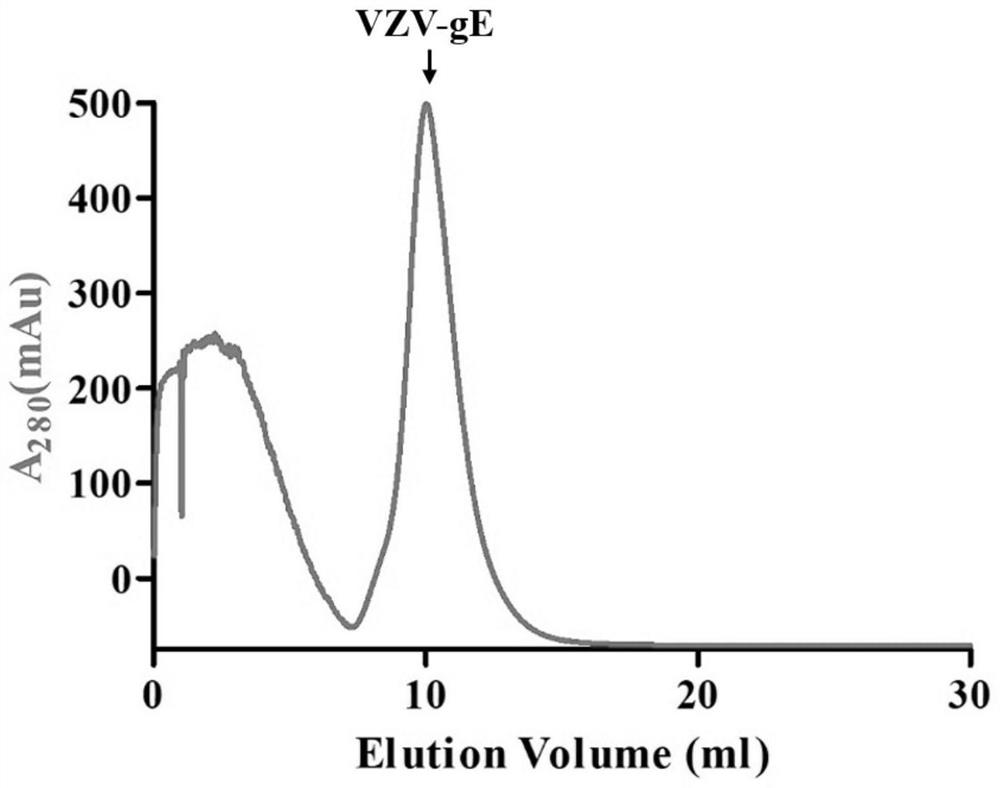
[0051] 2. Purification of recombinant VZV-gE protein
[0052] The purification steps of the recombinant VZV-gE protein include membrane filtration, dialysis, first nickel column purification, TEV protease digestion combined with dialysis, second nickel column purification, gel filtration chromatography and ultracentrifugal concentration.
[0053] a. Membrane f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com