Compositions and methods
A technology of composition and medicine, applied in the field of adenovirus vector
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0315] Example 1 - Viral vector vaccine against VZV
[0316] We have generated a viral vector vaccine against VZV.
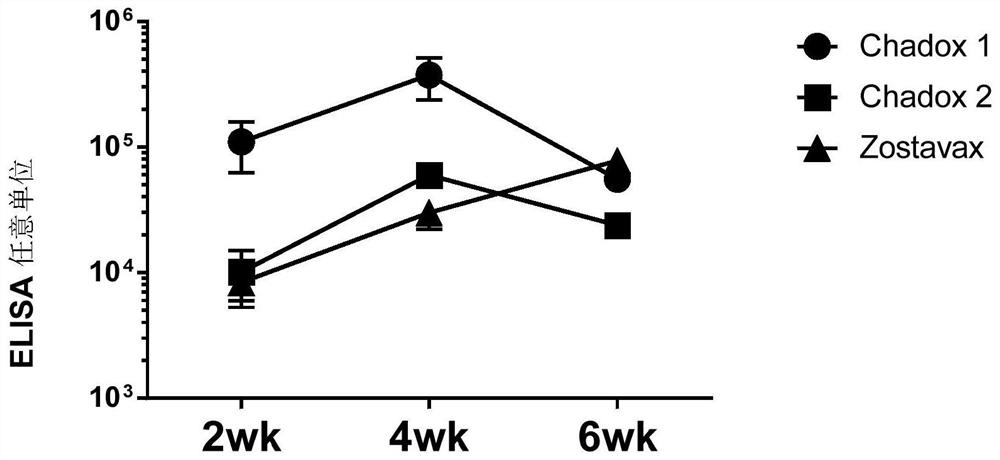
[0317] Our data show that the vaccine of the present invention is superior to the currently licensed prior art Zoster vaccine ( figure 1 ).
[0318] The higher CMI routinely achieved with viral vector vaccines may translate into higher efficacy compared with other vaccine modalities, and advantageously, viral vector vaccines require only Effectiveness is obtained with a single injection.
[0319] we refer to figure 1 .
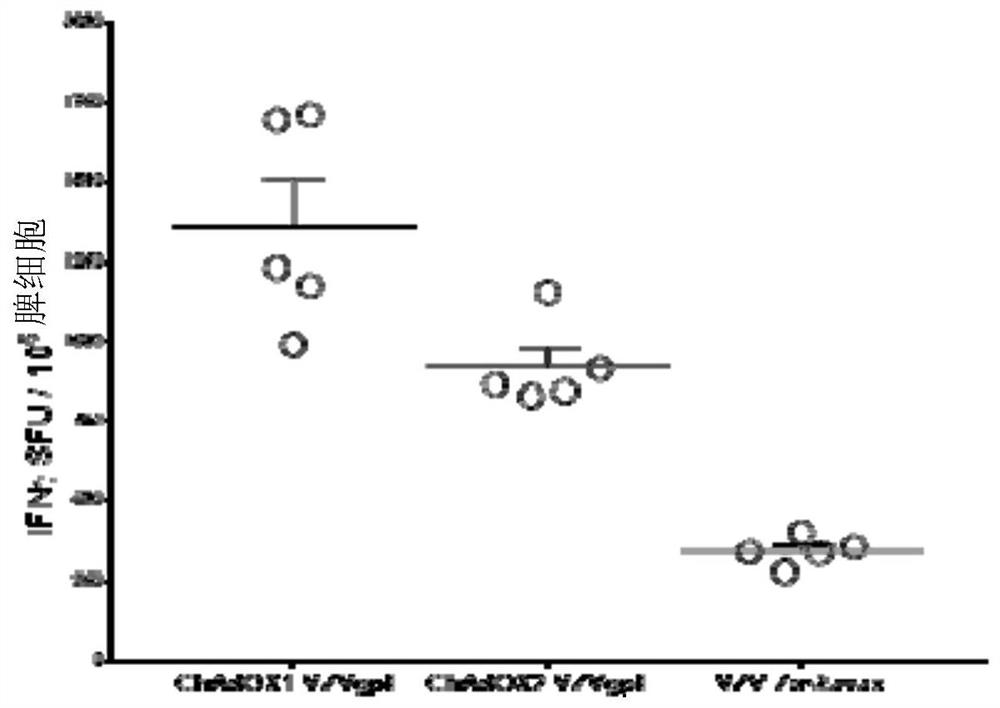
[0320] Use 11x 10 7 IU ChAdO X1 -VZVgpE or 1x 10 7 IU ChAdO X2- VZVgpE or 1.3x10 3 Balb / c mice in each group were inoculated intramuscularly with pfu Zotax (n=5). Splenocytes were harvested 2 weeks after final vaccination and cellular immune responses to peptides spanning the entire glycoprotein E were measured by ELISpot analysis.
[0321] ChAdO X1 - Response after VZV-gE was significantly higher than that after vaccination with Z...
example 2
[0349] Example 2: Effective expression of vectors of the present invention
[0350] We show western blot analysis of viral vector expression of VZVgpE. Subconfluent HEK293T (ChAdO X1 ). After 18 hours the cells were harvested, lysed, and the protein supernatant lysates were electrophoresed on a Biorad 4-12% gradient gel and probed with abcam 52549VZV diluted 1:1000 in 0.05% PBST to detect expression with ECL reagents .
[0351] The result is as Figure 7 shown. The swimlanes are as follows:
[0352] 0-molecular marker
[0353] 1 – ChAdO on HEK293T X1 VZVgpE MOI 1
[0354] 2 – ChAdO on HEK293T X1 VZVgpE MOI 5
[0355] 3 – ChAdO on HEK293T X2 VZVgpE MOI 1
[0356] 4 – ChAdO on HEK293T X2 VZVgpE MOI 5
[0357] 5 – VZV+ve control ~ 100ng
[0358] 6 – VZV+ve control ~ 500ng
[0359] It was thus demonstrated that the composition of the invention produces antigen expression in human cells.
example 3
[0360] Example 3: Immunogenicity of Viral Vectors Encoding Varicella-Zoster Virus Glycoprotein E
[0361] We demonstrate the cellular immunogenicity of a single-injection vaccine against VZV.
[0362] we refer to Figure 8 a. Use 11x 10 7 IU ChAdO X1 -VZVgpE or 1x 10 7 IU ChAdO X2 -VZVgpE or 1.3x 10 3 Balb / c mice in each group were inoculated intramuscularly with pfu Zotax (n=5). Splenocytes were harvested 2 weeks after final vaccination and cellular immune responses to peptides spanning the entire glycoprotein E were measured by ELISpot analysis.
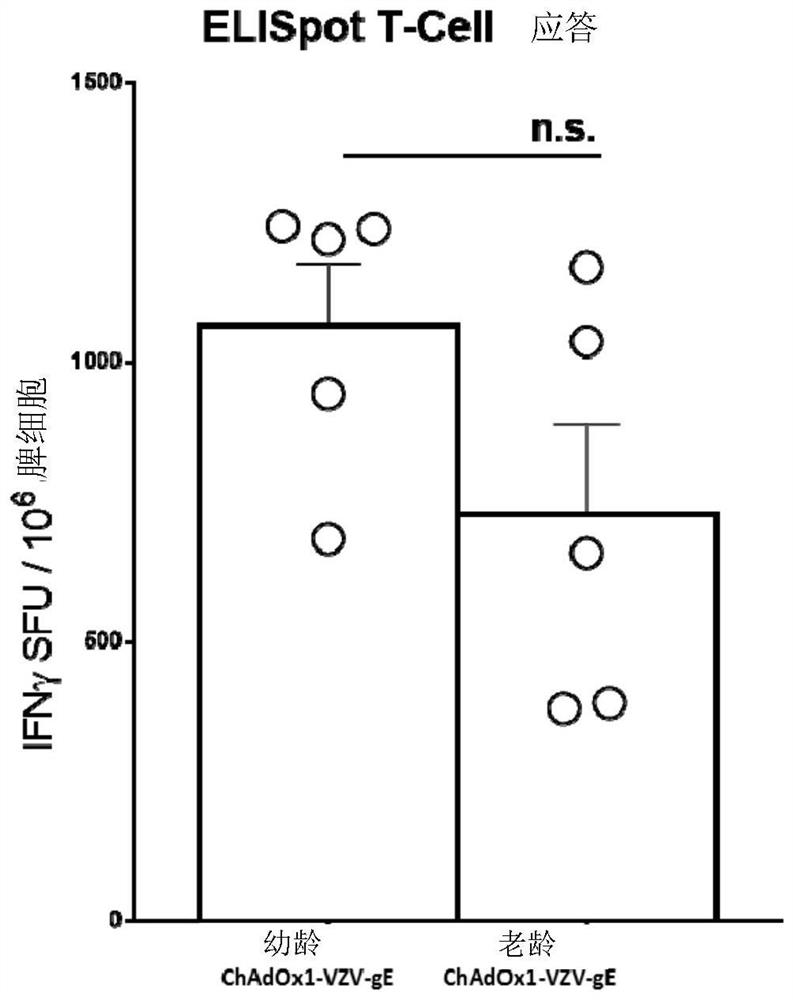
[0363] we refer to Figure 8 b. Use 1x 10 7 IU X1 -VZVgpE ("old mice" are pre-breeding mice, usually older than 24 weeks) or 1.3x 10 3 Groups of Balb / c mice (n=5, usually 8-10 weeks old unless otherwise stated) were inoculated intramuscularly with pfu Zotax. Splenocytes were harvested at indicated times after final vaccination and cellular immune responses against peptides spanning the entire glycoprotein E were measur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com