Method for producing novel freeze-dried live attenuated varicella vaccine
A technology of a live attenuated vaccine and a production method, which is applied in the production field of a new type of freeze-dried live attenuated varicella vaccine, can solve problems such as restrictions on production methods, and achieve the effects of rapid preparation, improved safety and stability, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A production method of a novel freeze-dried live attenuated varicella vaccine, the production method comprising the following steps:
[0028] 1) Recovery and subculture of human diploid cell lines;
[0029] Resuscitate MRC-5 cells: Take out the frozen MRC-5 cells from liquid nitrogen, thaw them immediately in a 37°C water bath, centrifuge at 1000rpm for 5min, discard the upper layer of frozen storage solution, add fresh culture medium, mix gently and set aside for 37 Cultivate at ℃ for 2-4 days until the cells cover a single layer; wherein, the culture medium is MEM solution containing 10% calf serum and 1% glutamine, and no antibiotics are added during the vaccine production process.
[0030] Digestion and subculture: When the cells grow into a uniform and dense monolayer, pour off the culture medium in the culture bottle, add trypsin digestion solution to digest until the cell attachment is loose, the cell edge rolls up and the interval between cells increases, then p...
Embodiment 2
[0050] This embodiment is a preferred solution on the basis of embodiment 1. The quality of the raw materials used is the same as that of embodiment 1, and the same part as embodiment 1 will not be repeated. Please refer to embodiment 1.
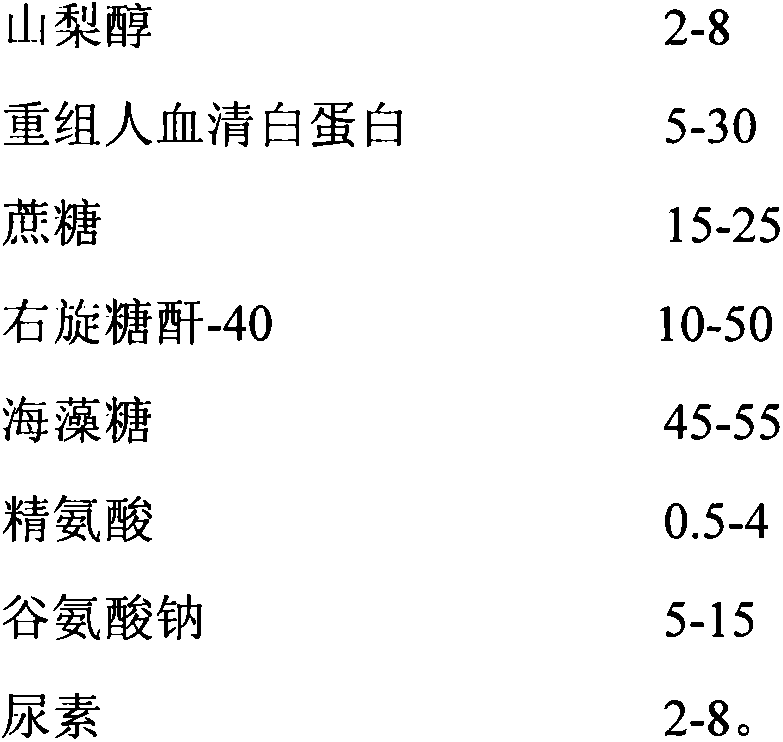
[0051] The raw materials of the lyoprotectant include sorbitol, recombinant human serum albumin, sucrose, dextran, trehalose, arginine, sodium glutamate and urea, and the ratio of parts by weight of each raw material is:
[0052]
[0053] The lyoprotectant is the 199 comprehensive culture medium containing the above-mentioned raw materials.
[0054] The recombinant human serum albumin described in this example is yeast-expressed recombinant human serum albumin Catalog No. PRO-332 produced by Prospec Company of Israel.
[0055] The microcarriers described in this example are Cytodex series microcarriers produced by General Electric Company of the United States; the stirred bioreactor produced by NBS Company of the United States is used in ...
Embodiment 3
[0058] This embodiment is a preferred solution on the basis of embodiment 1. The quality of the raw materials used is the same as that of embodiment 1, and the same part as embodiment 1 will not be repeated. Please refer to embodiment 1.
[0059] The raw materials of the lyoprotectant include sorbitol, recombinant human serum albumin, sucrose, dextran, trehalose, arginine, sodium glutamate and urea, and the ratio of parts by weight of each raw material is:
[0060]
[0061]
[0062] The lyoprotectant is the 199 comprehensive culture medium containing the above-mentioned raw materials.
[0063] The microcarriers described in this example are Fibra-Cel Disks series microcarriers produced by NBS Company of the United States; the packed bed bioreactor produced by Biou Company of Switzerland is used in this example.
[0064] Please refer to Table 1 for the titer, moisture analysis and stability test results of the varicella vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com