Influenza virus splitting vaccine and preparation method thereof
A technology of influenza virus and vaccine, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of influenza virus split vaccines that have not been reported in the literature, achieve high safety in use, and ensure public health Safe and good immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
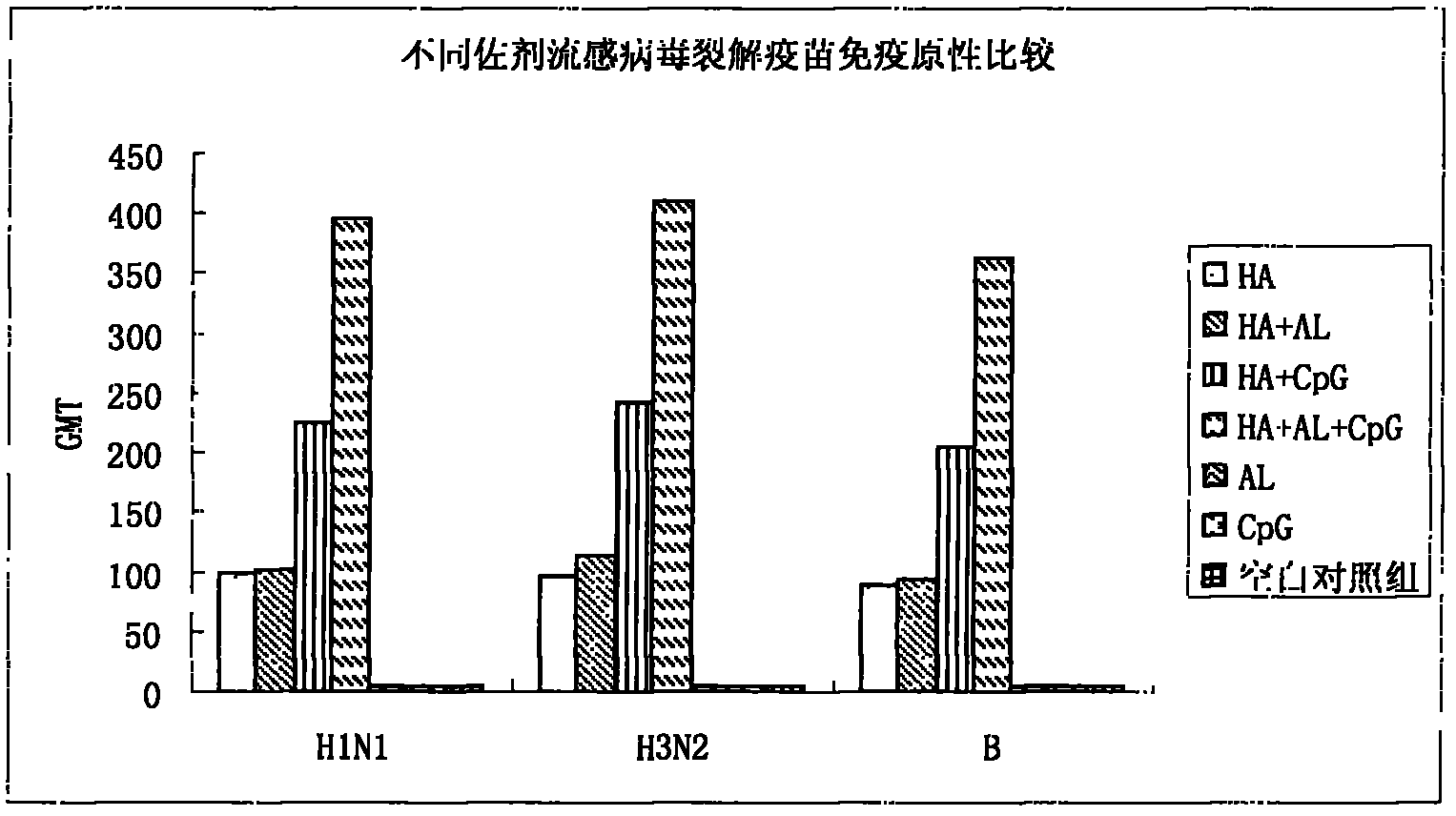
Image
Examples
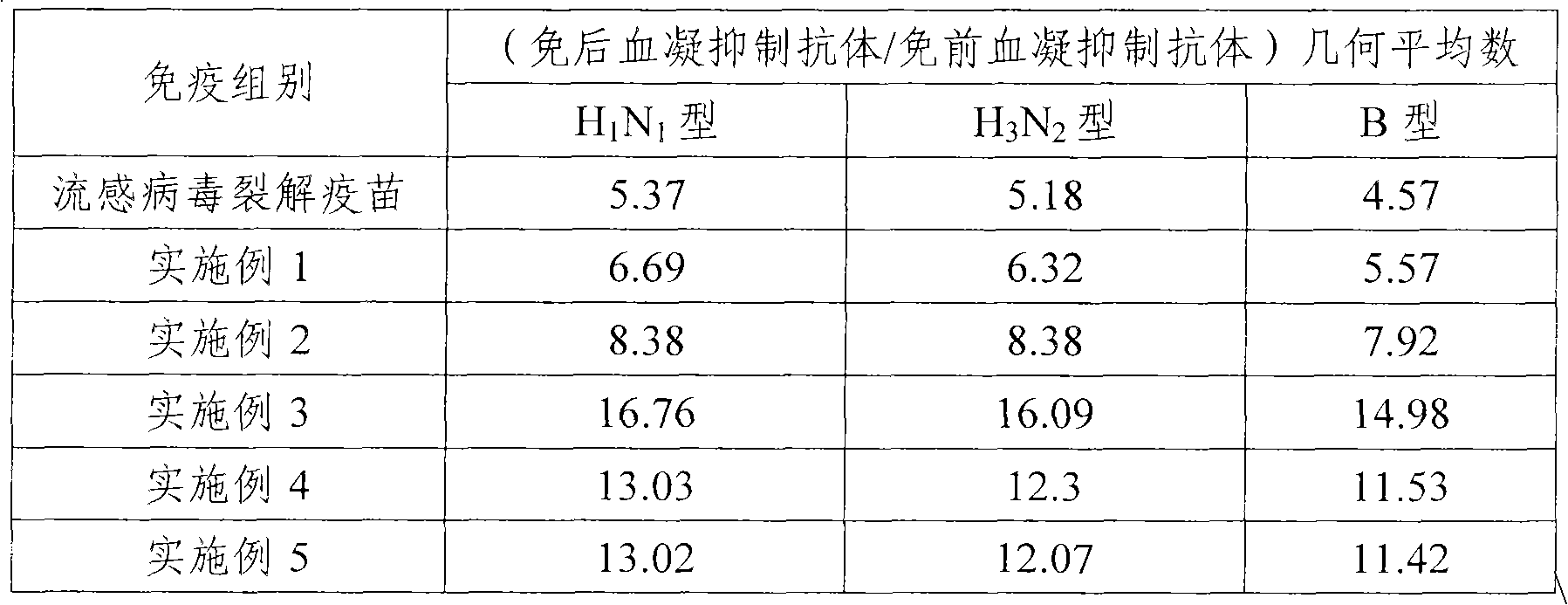
Embodiment 1
[0033] 1) Influenza virus proliferated in chicken embryos
[0034] H 1 N 1 、H 3 N 2 , Influenza B virus working seeds were inoculated in 2000 10-day-old healthy chicken embryo allantoic cavities respectively, placed at 34°C, and H 1 N 1 、H 3 N 2 , Type B cultured for 48, 48 and 72 hours respectively;
[0035] 2) Concentration by ultrafiltration
[0036] Place the chicken embryos in a 4°C freezer overnight, and collect H 1 N 1 、H 3 N 2 1. Type B influenza virus allantoic fluid, obtained 23500 milliliters of influenza virus liquid in total, the allantoic fluid was centrifuged at 6000 rpm for 20 minutes, and the supernatant was concentrated to 300 milliliters with an ultrafiltration membrane with a molecular weight cut-off of 300kD;
[0037] 3) centrifugal purification:
[0038] The influenza virus liquid sample concentrated by ultrafiltration was purified by sucrose density gradient centrifugation, monitored by 280nm ultraviolet light, and the virus liquid with viru...
Embodiment 2
[0047] 1) Influenza virus proliferated in chicken embryos
[0048] H 1 N 1 、H 3 N 2 , Influenza B virus working seeds were inoculated in 2000 10-day-old healthy chicken embryo allantoic cavities respectively, placed at 34°C, and H 1 N 1 、H 3 N 2 , Type B cultured for 48, 48 and 72 hours respectively;
[0049] 2) Concentration by ultrafiltration
[0050] Place the chicken embryos in a 4°C freezer overnight, and collect H 1 N 1 、H 3 N 2 1. Influenza virus type B allantoic fluid, 23000 milliliters of influenza virus liquid was obtained, the allantoic fluid was centrifuged at 6000 rpm for 20 minutes, and the supernatant was concentrated to 320 milliliters with an ultrafiltration membrane with a molecular weight cut-off of 300kD;
[0051] 3) Centrifugal purification: The influenza virus liquid concentrated by ultrafiltration is purified by sucrose density gradient centrifugation, monitored by 280nm ultraviolet light, and the virus liquid with virus peaks is collected as...
Embodiment 3
[0058] 1) Influenza virus proliferated in chicken embryos
[0059] H 1 N 1 、H 3 N 2 , Influenza B virus working seeds were inoculated in 2000 10-day-old healthy chicken embryo allantoic cavities respectively, placed at 34°C, and H 1 N 1 、H 3 N 2 , Type B cultured for 48, 48 and 72 hours respectively;
[0060] 2) Concentration by ultrafiltration
[0061] Place the chicken embryos in a 4°C freezer overnight, and collect H 1 N 1 、H 3 N 2 , Type B influenza virus allantoic fluid, obtained 22500 milliliters of influenza virus liquid altogether; The allantoic fluid was centrifuged at 6000rpm for 20 minutes respectively, and the supernatant was concentrated to 340 milliliters with an ultrafiltration membrane with a molecular weight cut-off of 300kD;
[0062] 3) Centrifugal purification: The influenza virus liquid sample concentrated by ultrafiltration was purified by sucrose density gradient centrifugation, monitored by 280nm ultraviolet light, and the virus liquid with v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com