Liposome influenza virus antigen vaccine and preparing method thereof
A technology for influenza virus and antigen vaccines, applied in liposome delivery, antiviral agents, pharmaceutical formulations, etc., can solve the difficulties of epitope vaccines in inducing multiple immune responses, clinical application limitations, and difficulty in eliciting strong immune responses, etc. problems, to achieve stable performance, long shelf life, and reduce usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
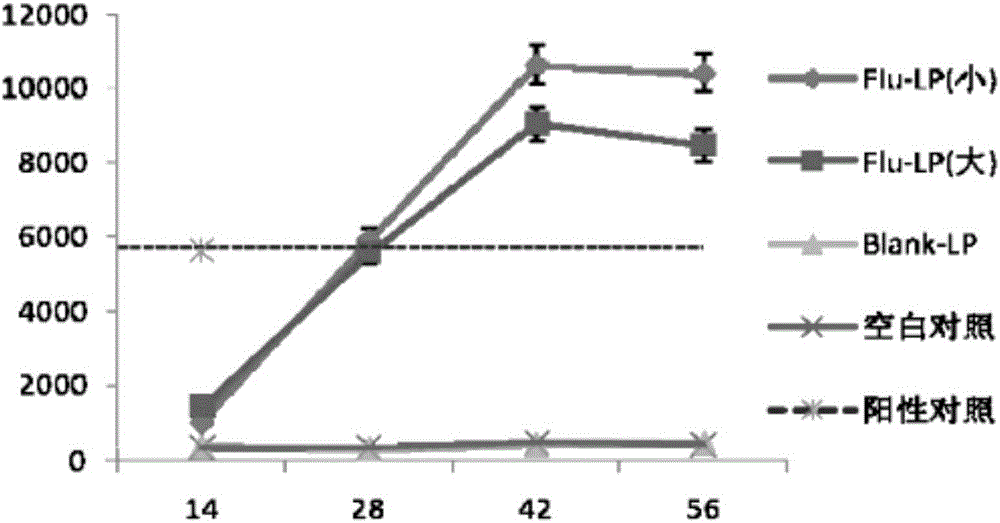
Image
Examples
preparation example Construction
[0037] (1) preparation of influenza virus antigen vaccine stock solution;
[0038] Take any one or more strains of influenza virus, inoculate them on the virus culture medium respectively, culture them at 33°C-35°C for 48-72 hours, extract the influenza virus body, inactivate it with formaldehyde, concentrate it by ultrafiltration, Nano collider lyses the virus and purifies it by column chromatography to make the stock solution of influenza virus antigen vaccine;
[0039] Among them, the influenza virus strains include the three most common influenza virus strains, Type A (H1N1), Type A (H3N2) and Type B, which makes the stock solution of influenza virus antigen vaccine more practical;
[0040] (2) Preparation of empty liposomes;
[0041] Phospholipids, cholesterol and antioxidants are mixed and dissolved in an organic solvent, and after vacuum rotary evaporation, membrane-shaped empty liposomes are obtained;
[0042] Wherein, phospholipids include one or more of soybean lec...
Embodiment 1
[0054] (1) Preparation of influenza virus antigen vaccine stock solution
[0055] Get three kinds of influenza virus strains (comprising type A 1, type A 3 and type B), three kinds of influenza viruses are inoculated on the virus culture medium respectively, preferably, 5-10 days old healthy chicken embryo is used as virus culture medium; After culturing at 33°C-35°C for 48-72 hours, harvest allantoic fluid virus, slowly add 0.15%-4% formaldehyde at 36°C and let stand for 24 hours, then stand at room temperature for 24 hours to complete inactivation; Then in a 100KD ultrafiltration centrifuge tube, under the centrifugation condition of 3000g (g is the acceleration of gravity), carry out ultrafiltration concentration, nano collider cracking virus and purification by Sephadex G-200 (Sephadex G-200) column chromatography Make trivalent virus subunit influenza virus antigen vaccine stock solution;
[0056] Each mL of influenza virus antigen vaccine stock solution contains the fol...
Embodiment 2
[0073] Compared with Example 1, except that the preparation process of empty liposomes is different, other preparation processes are the same.
[0074] In the present embodiment, the preparation process of empty liposome is:
[0075] Mix and dissolve 400mg soybean lecithin for injection, 150mg cholesterol, and 50mg vitamin E in 100ml chloroform, put the liquid in an eggplant-shaped bottle of a rotary evaporator, use a water bath at 60°C as the evaporation temperature, and vacuum rotary evaporation to obtain Membrane empty liposomes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com