Human anti-H7N9 avian influenza virus neutralizing antibody 1F7L and its use
A bird flu virus and antibody technology, applied in antiviral agents, antiviral immunoglobulins, applications, etc., can solve the problems of weakening the clinical efficacy of drugs and not being able to mediate cytotoxic killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
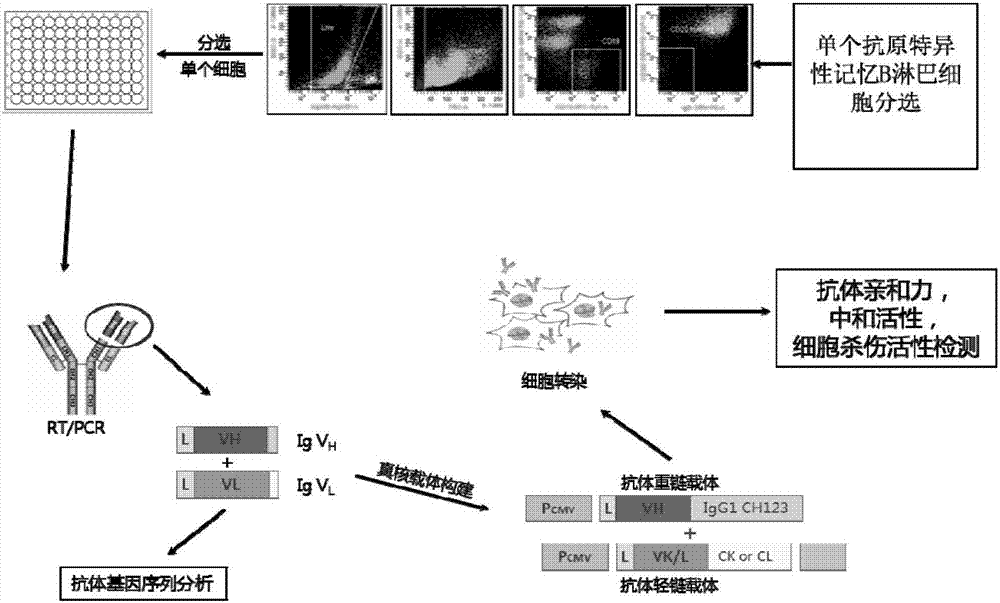
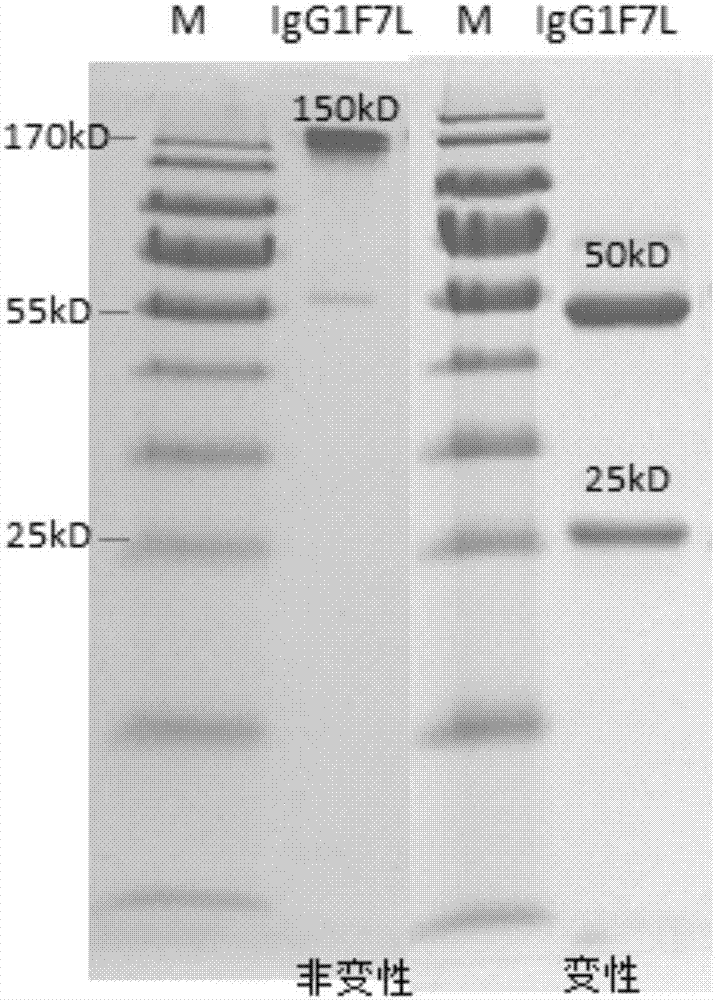
[0040] Example 1 Human-derived anti-H7N9 avian influenza virus neutralizing antibody 1F7L and its preparation method
[0041] 1. Antigen labeling
[0042] Purchase HA from Acrobiosystems (HA9-V5227) 7 protein, diluted to 0.5-2mg / ml with PBS, and then using Thermal company's biotin (EZ-LinkTMSulfo-NHS-LC-Biotin, 21335) according to its kit process to HA 7 Protein labeling (the ratio of the number of molecules is protein:biotin=1:20-100), incubate at room temperature in the dark for 0.5-2h, and then use a 10KD centrifugal semi-permeable column (MerckMillipore, UFC501096) to centrifuge 4-6 times at 8000g, and use Supplement with sterile PBS, remove excess biotin molecules, and mark HA 7 Protein molecules will be used to screen HA 7 specific memory B cells.
[0043] 2. Antigen-specific memory B cell sorting and reverse transcription
[0044] The convalescent peripheral blood mononuclear cells were isolated from patients with H7N9 infection, washed once with PBS, and then resu...
Embodiment 2
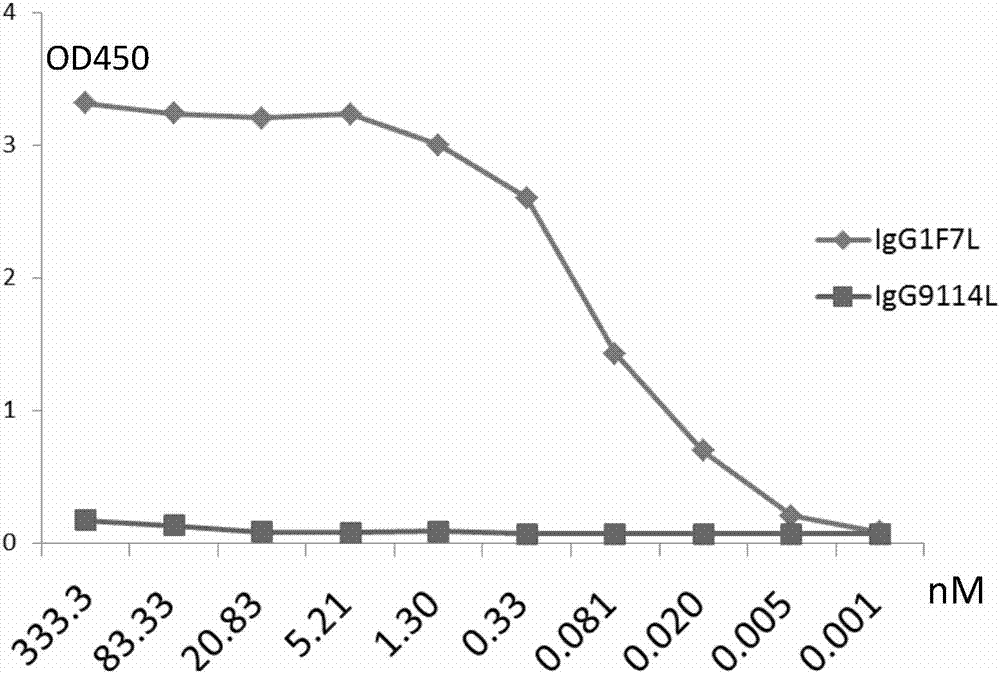
[0111] Embodiment 2 monoclonal antibody 1F7L is aimed at HA 7 protein EC 50 determination
[0112] Dilute HA with ELISA coating solution 7 The protein was adjusted to 1 μg / ml, and then 50 μl per well was coated on an ELISA plate (Corning, 3690) at 4° C. overnight. Plates were washed with PBST and blocked with PBS containing 5% skimmed milk for more than 2 hours. Starting from the initial concentration of 50 μg / ml, the monoclonal antibody was diluted 4-fold with PBS containing 5% skimmed milk, and then added to the blocked ELISA plate. A total of 10 gradients were set up, with two repetitions. The wells without antibodies were used as negative controls, and IgG9114L (ie CR9114, Dreyfus C, Laursen NS, Kwaks T, Zuijdgeest D, Khayat R, Ekiert DC, et al. Highlyconserved protective epitopes on influenza B viruses. Science 2012 Sep 14; 337(6100): 1343-8) is an antibody control. Goat anti-human IgG Fc-HRP (1:10000, Jackson immunolab, 109-036-098) as secondary antibody, 100μl TMB ...
Embodiment 3
[0113] Example 3 Determination of neutralizing activity of monoclonal antibody 1F7L against H7N9 (A / Shenzhen / SP17 / 2014 (H7N9))
[0114] Inoculate MDCK cells in a 96-well cell culture plate, culture until the cell density is about 70%-90%, wash twice with PBS and set aside; dilute the monoclonal antibody to be detected 3 times on a 96-well microtiter plate (starting at 100μg / ml), each antibody to be detected is repeated in 4 wells, 8 gradients; according to the TCID of the virus 50 Titer dilute the virus so that the diluted virus titer is 200TCID 50 / 100μl; mix 60μl of the diluted virus solution with 60μl of the diluted monoclonal antibody sample, and incubate in a 37°C incubator for 2 hours to fully act on the antigen and antibody; then take 100μl of the virus plasma mixture and put it into the washed 96-well MDCK cells, Infect in an incubator at ℃ for 1 hour, replace the virus liquid with 150 μl of MEM medium supplemented with TPCK trypsin, and store at 37℃ CO 2 Cultivate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com