H7N9 virus-resistant monoclonal antibody
A monoclonal antibody and antibody technology, applied in the fields of molecular biology and cellular immunology, can solve problems such as unfavorable development prospects, uncontrollable results, and complicated humanization process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
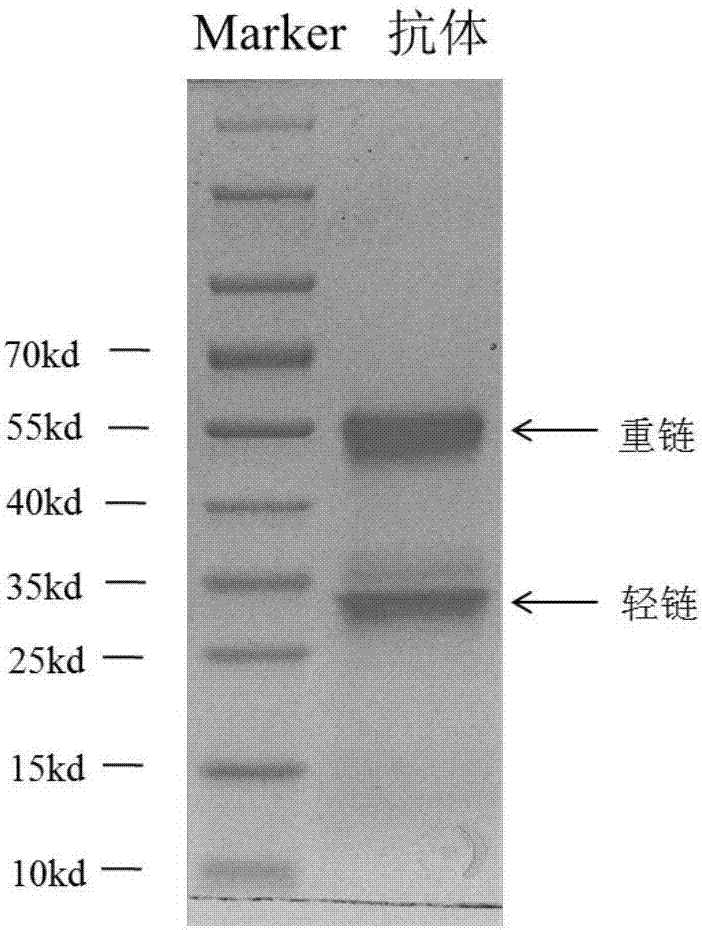
Image
Examples
Embodiment 1
[0101] Example 1 Preparation of monoclonal antibody mAb6137 against H7N9 virus
[0102] 1. Memory B cell sorting
[0103] 1.1 Isolation of PBMCs
[0104] Three samples of peripheral anticoagulant blood from H7N9 convalescent patients were collected, and PBMCs from peripheral blood were separated by Ficoll density gradient centrifugation and stored in liquid nitrogen.
[0105] 1.2 Acquisition of memory B cells
[0106] The purified HA protein was labeled with PE and APC fluorescent dyes, and then mixed with Anti-CD3-Cy7APC, Anti-CD14-FITC, Anti-CD20-Cy7PE, Anti-CD19-PE-CF594, Anti-IgG-BV421, Anti-IgM -Cy55 fluorescently labeled antibodies were mixed in corresponding proportions. Take out PBMC cells from liquid nitrogen, wash with PBS after thawing, first stain the dead cells with AQUA staining solution, and then incubate with the above-mentioned mixed antibody on ice to bind. Unbound antibodies were washed by centrifugation at 800g, washed with PBS and passed through a cell...
Embodiment 2
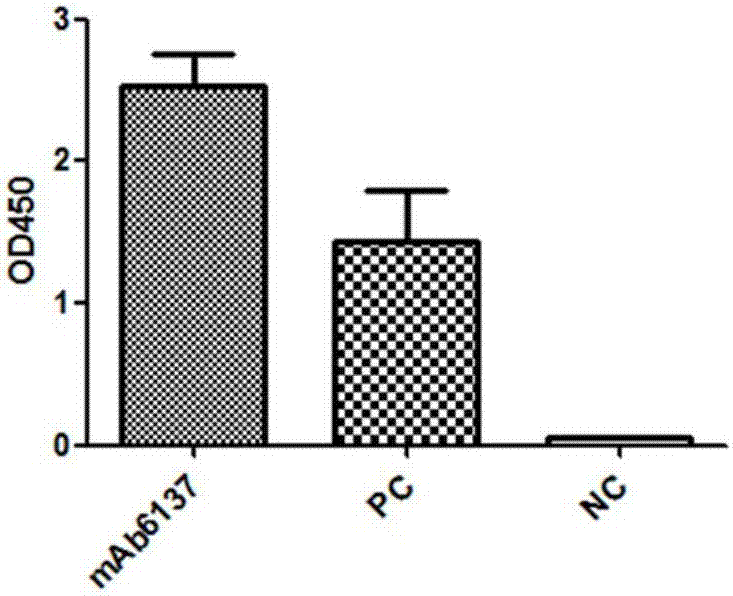
[0123] Example 2 Detection of mAb6137 Antibody Binding Specificity
[0124] Detection of binding of purified antibody to H7N9 virus HA protein by immunoblotting
[0125] 1. Steps:
[0126] (1) The HA protein of the purified H7N9 virus was subjected to SDS-PAGE, and the HA protein of the purified H3N2 virus was used as a control at the same time. After the electrophoresis was completed, the protein in the gel was transferred to a PVDF membrane, and the PVDF membrane was 5% skimmed milk 4 Block overnight at ℃, then add 50μg of purified antibody, incubate at 37℃ for 2h, and then elute with PBST;
[0127] (2) Add anti-human IgG secondary antibody at 1:2000, incubate at 37°C for 1 hour, and then elute with PBST;
[0128] (3) PVDF membrane plus chemiluminescent liquid is exposed in a dark room.
[0129] 2. Results
[0130] The result is as image 3 As shown, the purified mAb6137 antibody binds to the H7N9 virus HA protein. image 3 "1" represents the lane where the HA protein ...
Embodiment 3
[0131] Example 3 Hemagglutination inhibition experiment of mAb6137 antibody
[0132] 1. Treatment of serum and antibody
[0133] A positive control serum is the serum of a patient in the recovery period of H7N9 virus infection, and a negative control serum is the serum of a healthy adult. Add 3 times the volume of RDE to the serum and antibody, incubate at 37°C for 16-18 hours, inactivate at 56°C for 30 minutes before use, add 6 times the volume of PBS to the serum, and the final concentration is 1:10, without adding the antibody, the final concentration is 1:1: 4.
[0134] 2. Preparation of 1% turkey red blood cells
[0135] Inhale 2ml of Alfred's solution in the syringe in advance, and take 2ml of venous blood from the root of the turkey wing to form a 4ml whole blood mixture. Add 4ml of blood to a 50ml centrifuge tube, add cold PBS solution to the centrifuge tube to 50ml, mix gently and centrifuge at 2000rpm for 5 minutes to remove the supernatant suspension, then wash p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com