Humanized antibody combined with tetanus toxin heavy chain C-terminal structural domain and application
An anti-tetanus and toxin technology, applied in the fields of immunology and microbiology, can solve the problems of easily causing allergic reactions, spreading infectious diseases, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
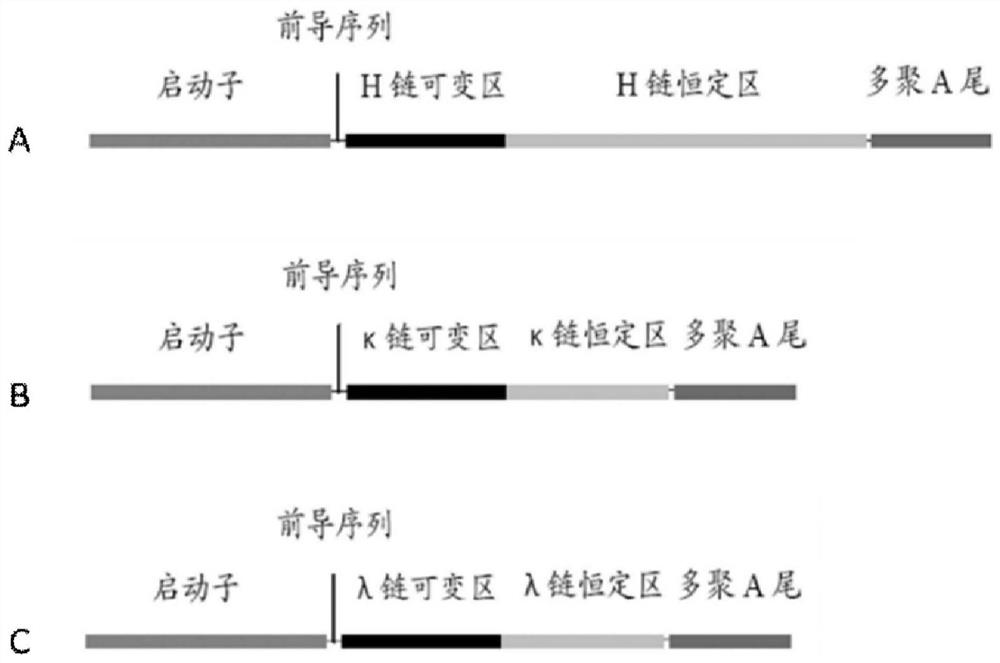
Image
Examples
Embodiment 1
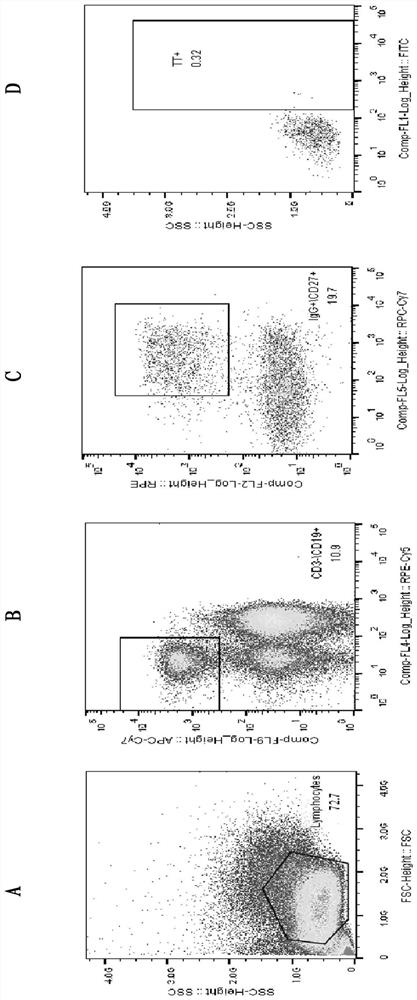
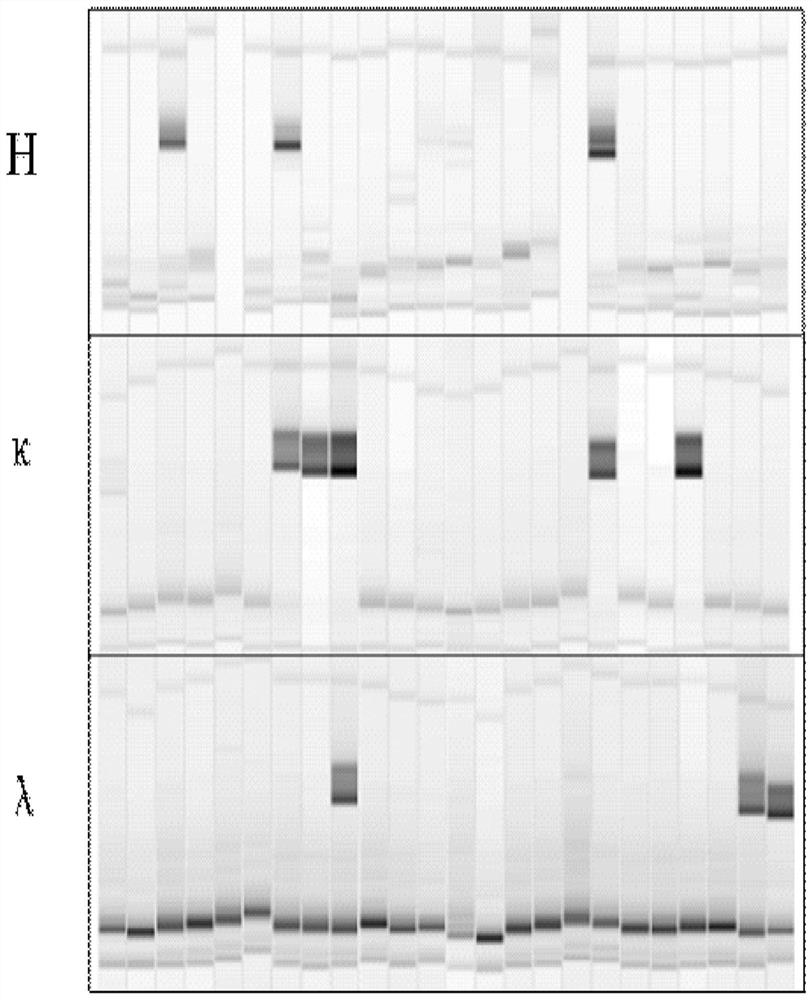
[0031] Example 1. Screening and preparation of monoclonal antibodies
[0032] 1. Blood Sample Collection
[0033] After obtaining informed consent, the recombinant tetanus vaccine (the main component is TeNT-H, the C-terminal domain of recombinant tetanus toxin heavy chain C , GenBank: AF154828) 10 mL of blood sample 28 days after the second immunization of subjects in the clinical trial was used for follow-up experiments.
[0034] 2. FITC-labeled Tetanus Toxoid (TT)
[0035] Specific memory B cells need to be sorted by fluorescently labeled antigens, and the method of FITC-labeled tetanus toxoid (TT) is as follows:
[0036] 1) Fluorescein Isothiocyanate_FITC (SIGMA, F4274) was dissolved in DMSO at a concentration of 20 mg / mL.
[0037]2) Take 100 μL of 3.3 mg / mL TT solution, add pH 9.6 carbonate buffer to 400 μL.
[0038] 3) Add 8 μL FITC to the TT solution, and incubate at 4° C. in the dark for 3 hours.
[0039] 4) Use a 50kDa ultrafiltration tube to change the solution ...
Embodiment 2
[0123] Example 2.ELISA detection of antibody binding activity
[0124] 1. The day before the experiment, the 96-well enzyme-linked plate was coated with 1 μg / mL tetanus toxoid TT (purchased from China Food and Drug Control Institute) and tetanus toxin heavy chain C-terminal domain (TeNT-H C , GenBank: AF154828), 100 μL per well for coating. Put the coated ELISA plate in a humid box overnight at 4°C.
[0125] 2. Wash 5 times with a plate washer on the day of the experiment. Add 100 μL of blocking solution to each well and place at room temperature for 1 hour.
[0126] 3. Wash the plate 5 times. Add 150 μL of T9-6 monoclonal antibody with a concentration of 3.3 μg / mL to the first well, and add 100 μL of diluent to the remaining wells. Aspirate 50 μL from the first well and add to the second well, and so on, dilute the solution in a 1:3 gradient to a final volume of 100 μL in each well. Let stand at room temperature for 1 hour.
[0127] 4. Wash the plate 5 times. The HPR-l...
Embodiment 3
[0131] Example 3. Mice challenge protection experiment
[0132] To evaluate the neutralizing toxin effect of T9-6 in mice, the evaluation method is as follows:
[0133] 1. Mice: BALB / c, 10 in each group, half male and half male, 6-8 weeks.
[0134] 2. Sample preparation: boric acid buffer: 8.5g NaCl, 4.5g boric acid, 0.5g sodium tetraborate decahydrate, add water to 1L, 0.22μm filter sterilization, pH 7.4; dilute tetanus toxin with boric acid buffer, in LD in mice 50 It was 15.8ng / kg.
[0135] 3. T9-6 administration group: 10 μg antibody (diluted in 50 μL PBS) + 2LD 50Tetanus toxin (total system 0.5mL); control group: 50μL PBS+2LD 50 Tetanus toxin (total system 0.5mL), incubated at 37°C for 1 hour.
[0136] 4. Intraperitoneally inject the toxin / antibody mixture into mice, 0.5 mL per mouse.
[0137] 5. Observe for 10 days.
[0138] Results: All the mice in the control group died within 2 days, while the mice in the T9-6 administration group could die within 2LD 50 100% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com