Angiotensin II receptor 1 type polypeptide-vector vaccine and application thereof
An angiotensin, carrier vaccine technology, applied in cardiovascular system diseases, antibody medical ingredients, medical preparations with non-active ingredients, etc. Poor treatment compliance and other problems, to achieve good target organ protection and lower blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
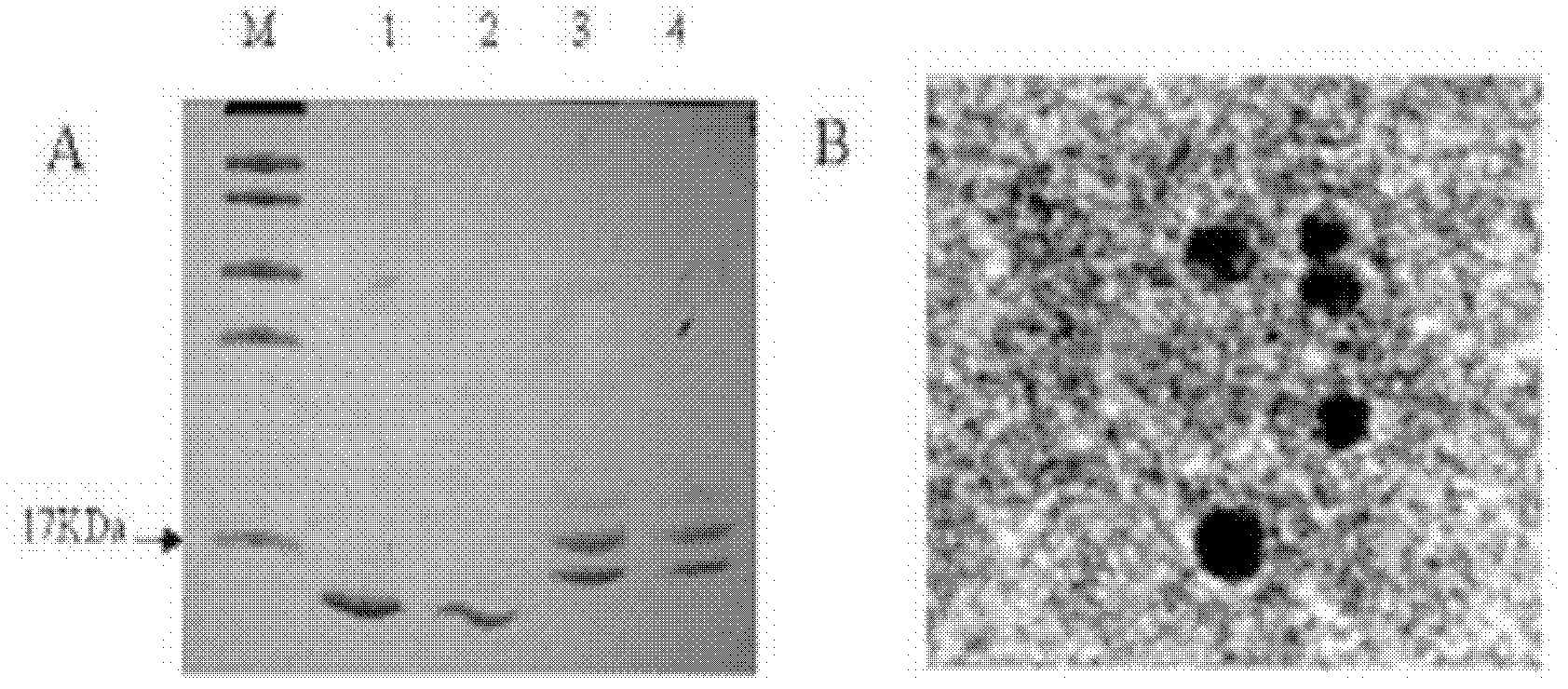
[0033] Implementation Case 1 Preparation and Identification of ATRQβ-001 Vaccine
[0034] The short peptide CGGAFHYESQ is directly synthesized by Shanghai Gil Biochemical Co., Ltd., and the purity of the short peptide is 95%.
[0035] The vector Qβ-2aa virus-like particle was constructed, expressed and purified by our laboratory, with a purity of more than 90%. It was prepared according to the following method:
[0036] 1) After the stop codon of Qβ bacteriophage CP protein gene was mutated from TGA to strong stop codon TAA, it was cloned into the prokaryotic expression vector pET28a(+), and the CP protein particle pETQβ-CP was obtained;
[0037] 2) In the Qβ phage CP elongation protein gene encoding the position of the immunodominance determining region, that is, between the 72nd and 73rd codons of the Qβ bacteriophage CP elongation protein gene, insert the nucleotide AAGCTT encoding lysine and leucine, and The stop codon of the CP protein gene was mutated from TGA to GGA an...
Embodiment example 2
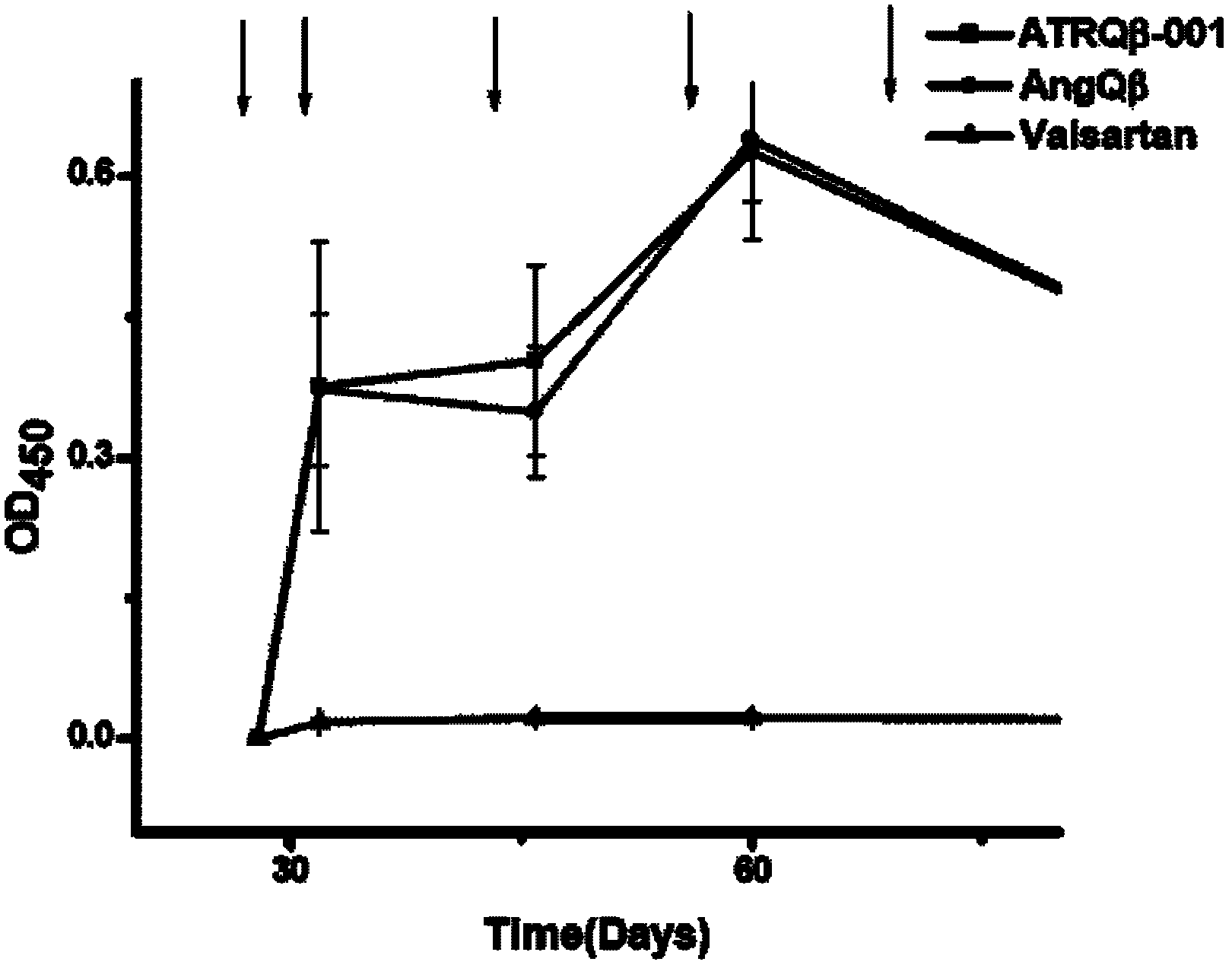
[0048] Implementation Case 2 Anti-ATRQβ-001 Vaccine Short Peptide Antibody Titer
[0049] Male spontaneously hypertensive rats came from the Shanghai Slack Experimental Animal Center, and the animals were raised in the SPF Animal Experiment Center of Tongji Medical College, using 12h / 12h light and non-restricted ordinary diet. Specifically divided into 4 groups: ATRQβ-001 vaccine immunization group; Ang II-Qβ vaccine immunization group (Ang II derivative and Qβ-2aa virus-like particle coupled vaccine); valsartan drug (Novartis, Switzerland) gavage group; PBS control SHR group. Except for the 8-week-old valsartan drug administration group, the other groups were all 6-week-old male SHR rats, with 9 rats in each group.
[0050] Take an equal volume of vaccine (not enough to make up with PBS) and PBS, inject subcutaneously at 3-4 points on the back of the rats, 400ul / rat, the vaccine dose of the vaccine group is about 300ug / rat, and boost twice every two weeks after the initial i...
Embodiment example 3
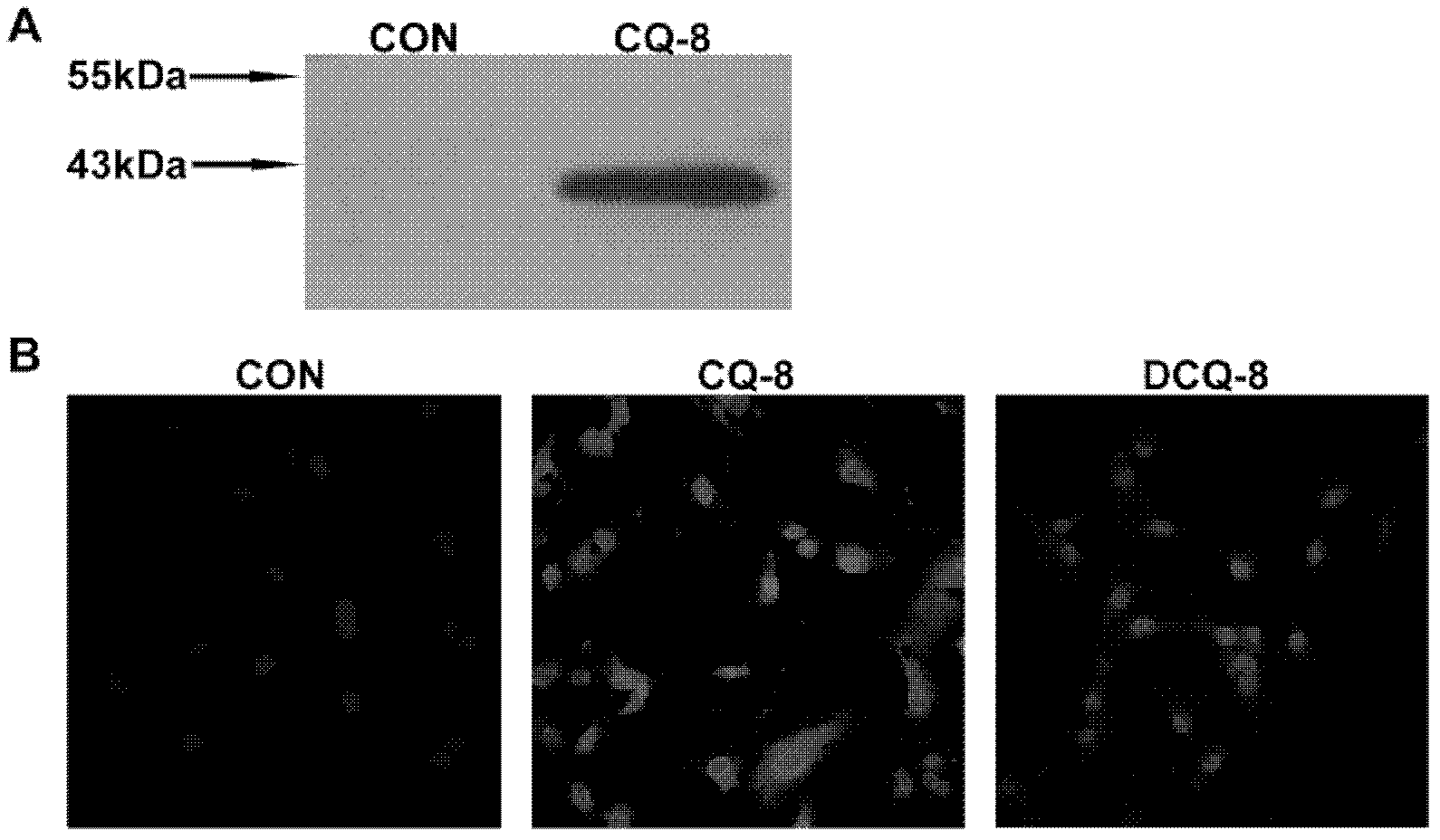
[0051] Implementation Case 3 Specificity of Anti-ATRQβ-001 Vaccine Short Peptide Antibody
[0052] The anti-peptide antibody CQ-8 of ATRQβ-001 vaccine was purified, and the specificity of ATRQβ-001 anti-peptide antibody CQ-8 was identified by immunoblotting and cellular immunofluorescence. Three groups were set up: CQ-8 was the anti-peptide antibody of animals immunized with ATRQβ-001 vaccine, DCQ-8 was the mixture after CQ-8 was neutralized by ATR-001 short peptide, and CON was the negative control antibody. Extract SD rat mesenteric tertiary arterial smooth muscle cell total protein, carry out immunoblotting analysis, the result shows that CQ-8 can combine with the protein of about 41kDa size in this total protein extract, and this protein has the same molecular weight as rat AT1A receptor (approximately 41kDa) consistent ( image 3 A). At the same time, it was found by cellular immunofluorescence that CQ-8 can bind to the AT1A receptor on the surface of rat mesenteric ter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com