Kit for fluorescent PCR (Polymerase Chain Reaction) detection of high-risk HPV (Human Papilloma Virus)
A technology for detection kits and kits, which are used in the determination/inspection of microorganisms, microorganisms, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This embodiment provides a detection kit capable of detecting 15 high-risk human papillomaviruses, which at least consists of the following components:
[0084] ① Nucleic acid release agent: Surfactin 0.01-0.5mmol / L (mass / volume), potassium chloride (KCl) 50-200mmol / L (mass / volume), sodium dodecylsulfonate (SDS) 0.01%~2% (mass / volume), ethanol 0.05%~1% (volume / volume);
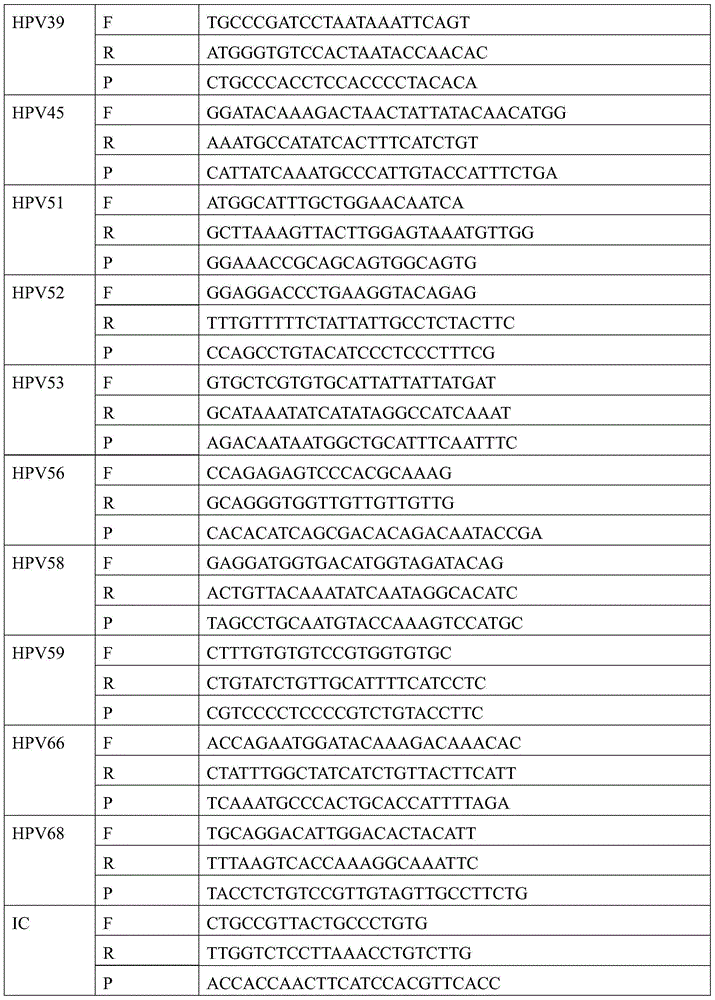
[0085]②PCR reaction solution: 5 μl of 10×PCR reaction buffer, 0.05mmol / L~0.2mmol / L deoxyribonucleoside triphosphate, 0.1μmol / L~0.3μmol / L upstream and downstream primers for target polynucleotide amplification HPV16-F and HPV16-R, HPV18-F and HPV18-R, HPV31-F and HPV31-R, HPV33-F and HPV33-R, HPV35-F and HPV35-R, HPV39-F and HPV39-R, HPV45- F and HPV45-R, HPV51-F and HPV51-R, HPV52-F and HPV52-R, HPV53-F and HPV53-R, HPV56-F and HPV56-R, HPV58-F and HPV58-R, HPV59-F and HPV59-R, HPV66-F and HPV66-R, HPV68-F and HPV68-R; 0.1 μmol / L~0.3 μmol / L probes for target polynucleotide detection HPV16-P, HPV18-P, ...
Embodiment 2
[0094] The operation steps of using the detection kit in Example 1 to detect high-risk HPV-DNA in wart surface exfoliated cells, women's cervical epithelial cells, and reproductive tract secretions are as follows:
[0095] 1. Prepare reagents in the reagent preparation area
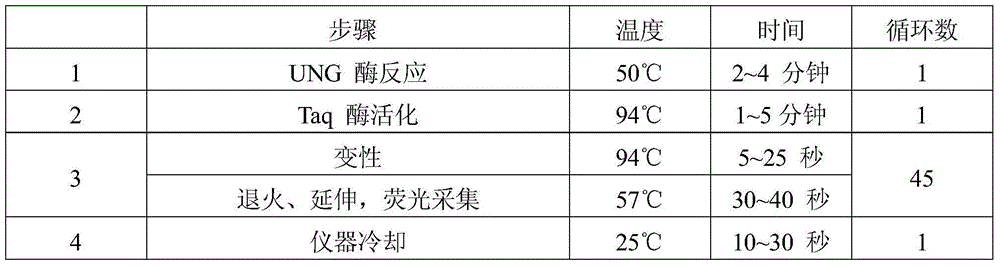
[0096] According to the number of samples to be tested, negative control, positive control, and quantitative reference products A~D, take the corresponding amount of PCR reaction solution (38~44μl / person) and enzyme mixture (1~2μl / person) in proportion and mix thoroughly. Homogenize PCR-mix, centrifuge briefly and set aside.
[0097] 2. Sample processing in the sample processing area
[0098] 1) Add 2-5 μl of nucleic acid release agent into each PCR reaction tube (it is recommended to inhale deeply and shallowly to avoid air bubbles), and add the sample to be tested, negative control, positive control, and quantitative reference product to different PCR reaction tubes. 3~5μl;
[0099] 2) At an interval...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com