Human papilloma virus infection gene amplification fluorescent detection kit
A fluorescent labeling and base technology, applied in the direction of microorganism-based methods, microorganism measurement/inspection, biochemical equipment and methods, etc., can solve the problems of inability to observe the relationship of HPV load, easy contamination of false positives, and inability to quantify, etc., to achieve Easy to popularize, high sensitivity and specificity, easy to accept the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The present invention will be described in detail below through examples.
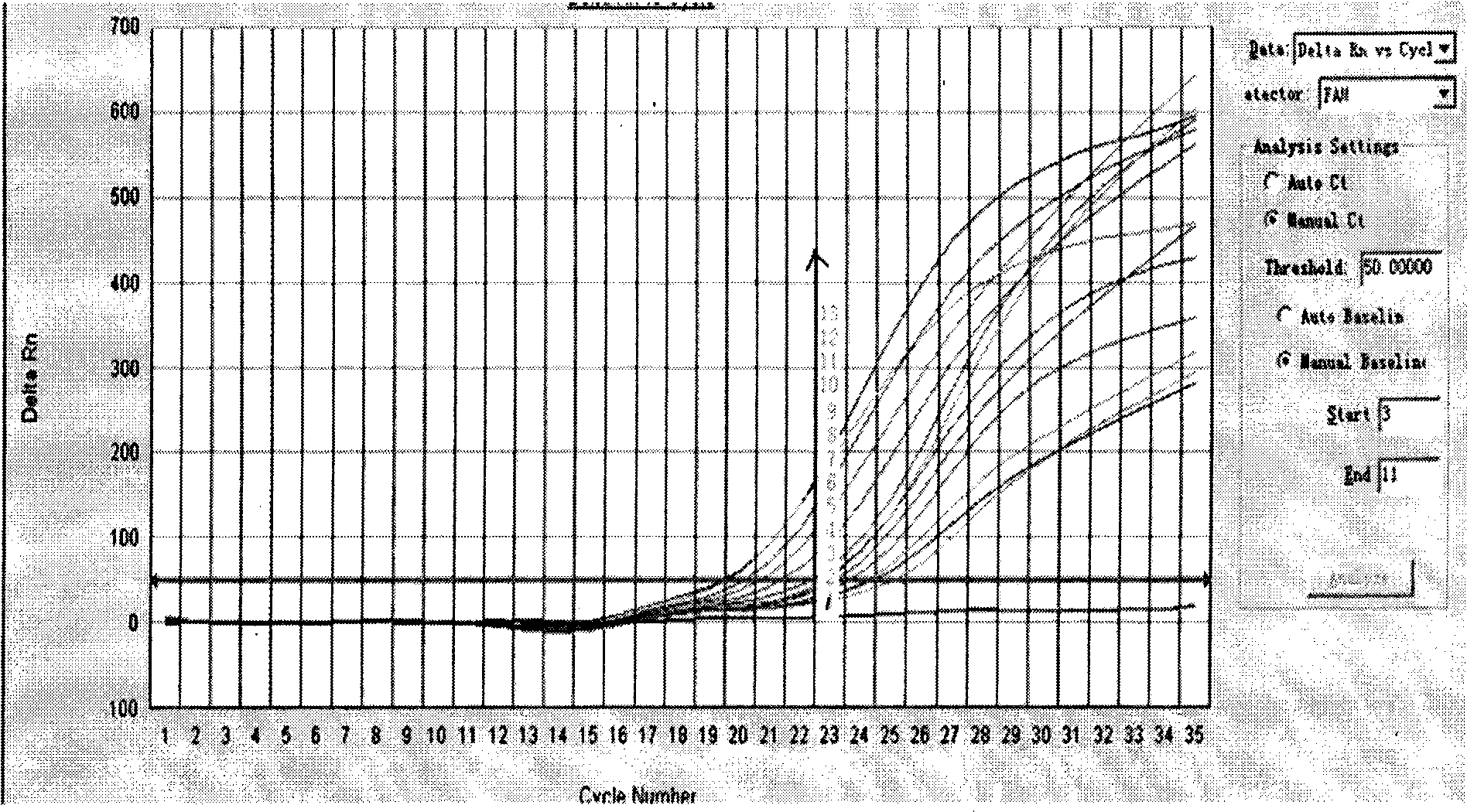
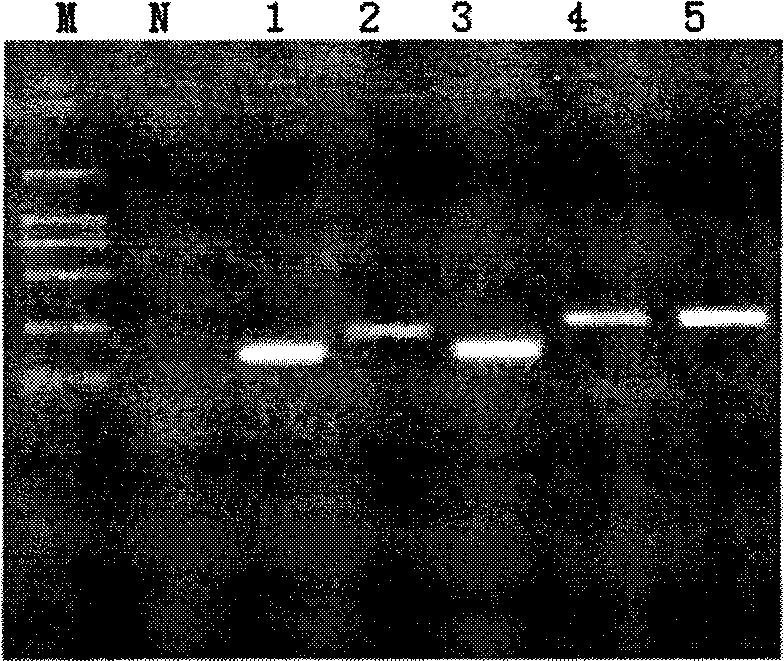
[0028] The present inventor prepared DNA complementary to 13 high-risk HPVs (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) respectively by analyzing the DNA of clinical samples. The probes of SEQ ID Nos: 1-13 and the primers of SEQ ID Nos: 14-39 are used to detect HPV infection and identify HPV high-risk types.
[0029] The nucleic acid amplification (PCR) fluorescent identification kit of high-risk human papillomavirus (HPV) infection provided by the present invention includes the above-mentioned probes and primers, DNA polymerase, and also includes PCR MIX, which includes all PCR reactions except the template Required reagents, as well as HPV53 plasmid for positive control and ultrapure water for negative control.
[0030] The steps of using the kit of the present invention are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com