HPV (human papilloma virus) high-risk typing fluorescence PCR (polymerase chain reaction) detection kit
A detection kit and the technology of the kit, applied in the field of human papillomavirus high-risk type typing fluorescent PCR detection kit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This embodiment provides a detection kit capable of detecting 15 high-risk human papillomaviruses and accurately typing them, which at least consists of the following components:
[0084] ① Nucleic acid release agent: Surfactin 0.01-0.5mmol / L (mass / volume), potassium chloride (KCl) 50-200mmol / L (mass / volume), sodium dodecylsulfonate (SDS) 0.01%~2% (mass / volume), ethanol 0.05%~1% (volume / volume);
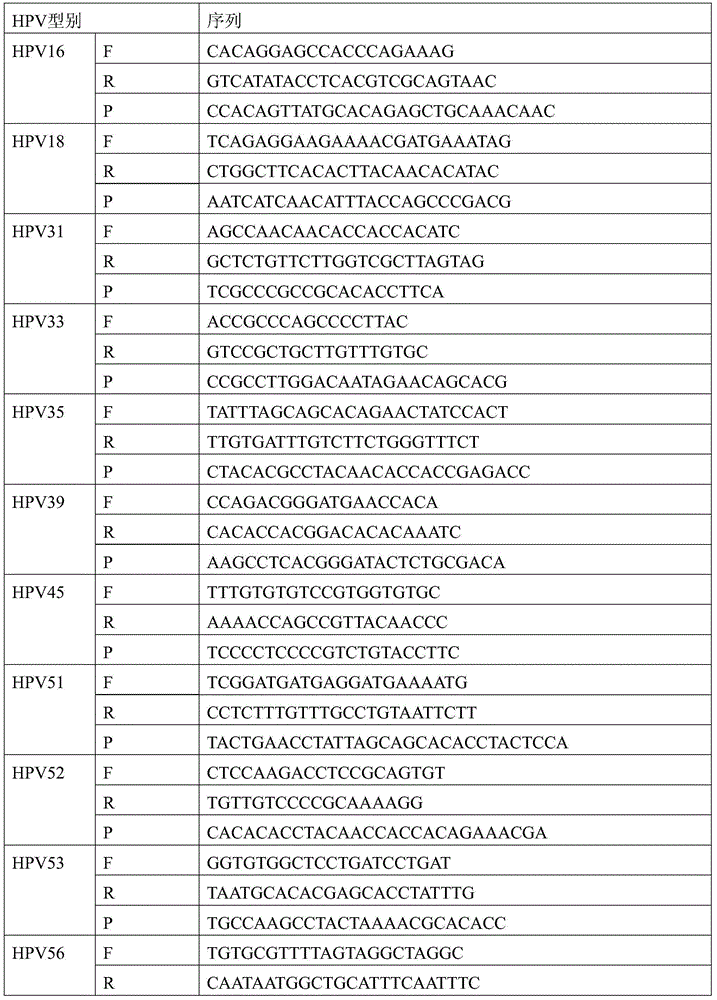
[0085]②PCR reaction solution: the first PCR reaction solution containing HPV16 and 18 primer probe sequences, used to detect HPV16 and 18 types; the second PCR reaction solution containing HPV39 and 31 primer probe sequences, used For the detection of HPV39 and 31 types; the third PCR reaction solution containing HPV33 and 58 primer probe sequences, used to detect HPV33 and 58 types; the fourth PCR reaction solution containing HPV45 and 59 primer probe sequences Reaction solution for detecting HPV45 and 59 types; the fifth PCR reaction solution comprising HPV51 and 66 primer ...
Embodiment 2
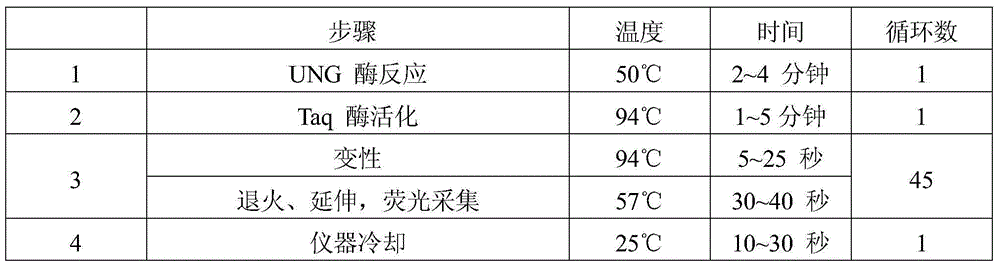
[0094] The operation steps of using the high-risk human papillomavirus nucleic acid typing detection kit in Example 1 to detect high-risk HPV-DNA in unknown samples such as wart surface exfoliated cells, women's cervical epithelial cells, and reproductive tract secretions are:
[0095] 1. Reagent preparation
[0096] Take out each component in the kit, place it at room temperature, wait for its temperature to balance to room temperature, mix well and set aside;
[0097] Take n parts of 8 kinds of HPV PCR reaction solutions, each part is 38-44 μl, and 8×n parts of enzyme mixture, each part is 1-2 μl, mix well to make PCR-mix, centrifuge briefly and set aside. Wherein, n=number of samples to be tested+2 quality control substances, and the quality control substances are negative control and positive control. In addition, since the PCR reaction solution containing HPV16 and 18 type primer probes needs to be used as a quantitative reference product, n+4 copies of this PCR reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com