Anti-HPV (human papilloma virus) antibody and preparation method and application thereof
A HPV16 and antibody technology, applied in the field of immunotherapy, can solve the problems of low complex efficiency, interference of target monoclonal antibody screening and epitope identification, cumbersome method process, etc., and achieve high specificity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The method for preparing antibodies of the present invention may further include purifying the collected antibodies.
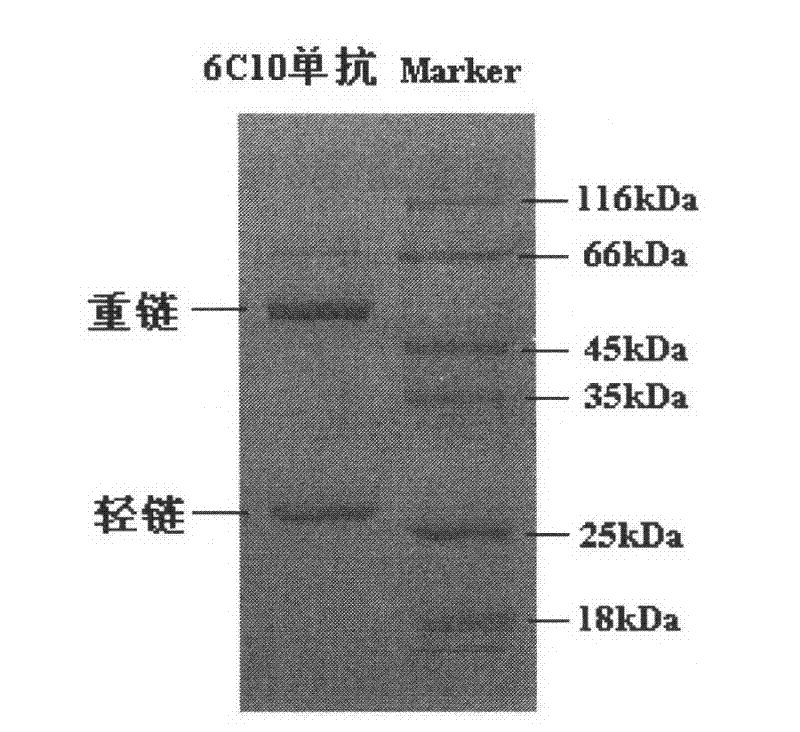
[0050] The present invention also provides a cell that produces an antibody of the present invention. In one embodiment, it is a 6C10 cell preserved in the General Microorganism Center of China Committee for Culture Collection of Microorganisms (CGMCC) with a preservation number of CGMCC 4144.
[0051] Purification of the antibodies of the present invention can be performed by conventional purification methods known to those of ordinary skill in the art, such as affinity chromatography, ion exchange chromatography, hydrophobic chromatography, molecular sieve chromatography, and the like.
[0052] The antibody of the present invention can be used to prevent or treat HPV16-infected diseases, including administering an effective amount of the antibody of the present invention to a subject, and a particularly preferred antibody is a monoclonal antibody produ...
Embodiment 1
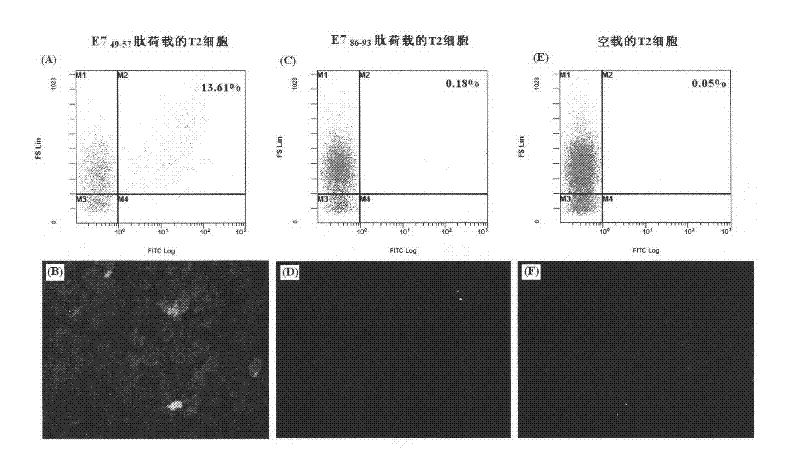
[0055] Example 1: Preparation and immunization of immune complexes of E7 protein 49-57 peptide
[0056] 49-57 peptide of HPV16 virus E7 protein, amino acid sequence: RAHYNIVTF; molecular weight 1119Da, theoretical isoelectric point 9.04. The peptide was synthesized by Jill Biochemical Company with a purity greater than 95%. Synthetic double-stranded RNA——PolyIC was used as an adjuvant, which was purchased from Sigma (Cat. No.: P9582).
Embodiment 2
[0058] Embodiment 2: the detection of serum antibody titer
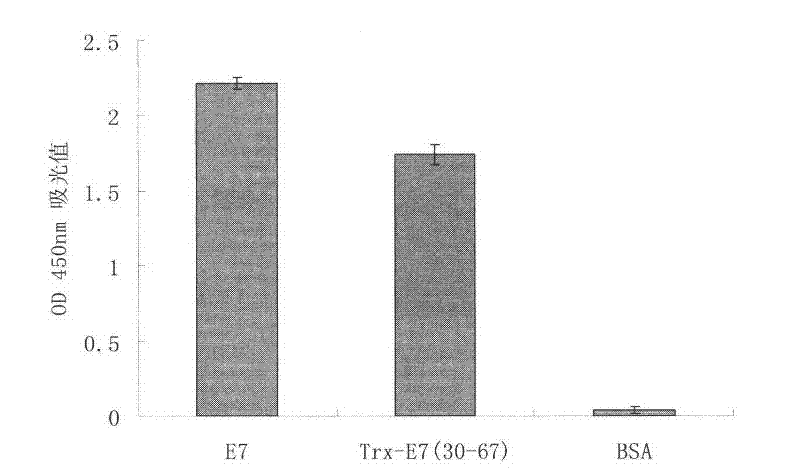
[0059] For the mice immunized as described in Example 1, a small amount of serum was collected from the tail vein on the 35th day after the initial immunization, and the serum antibody titer was detected by direct enzyme-linked immunoassay (ELISA).
[0060] The steps for detecting serum antibody titer by ELISA are as follows:
[0061] 1. First use the HPV16E7 protein (the preparation method of the protein is: synthesize the full-length HPV 16E7 gene, then express the E7 fusion protein with the Trx tag in the pET32a vector and BL21(DE3) host, and then use enterokinase to remove the Trx tag HPV16E7 protein can be obtained by dropping), and the amount of 1 μg per well was coated on a 96-well plate. Incubate the coated plate at 4°C for 16 hours, wash each well 6 times with washing buffer (PBS solution containing 0.05% Tween-20), add 100 μl of blocking solution (PBS solution containing 5% skimmed milk powder) to each wel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com