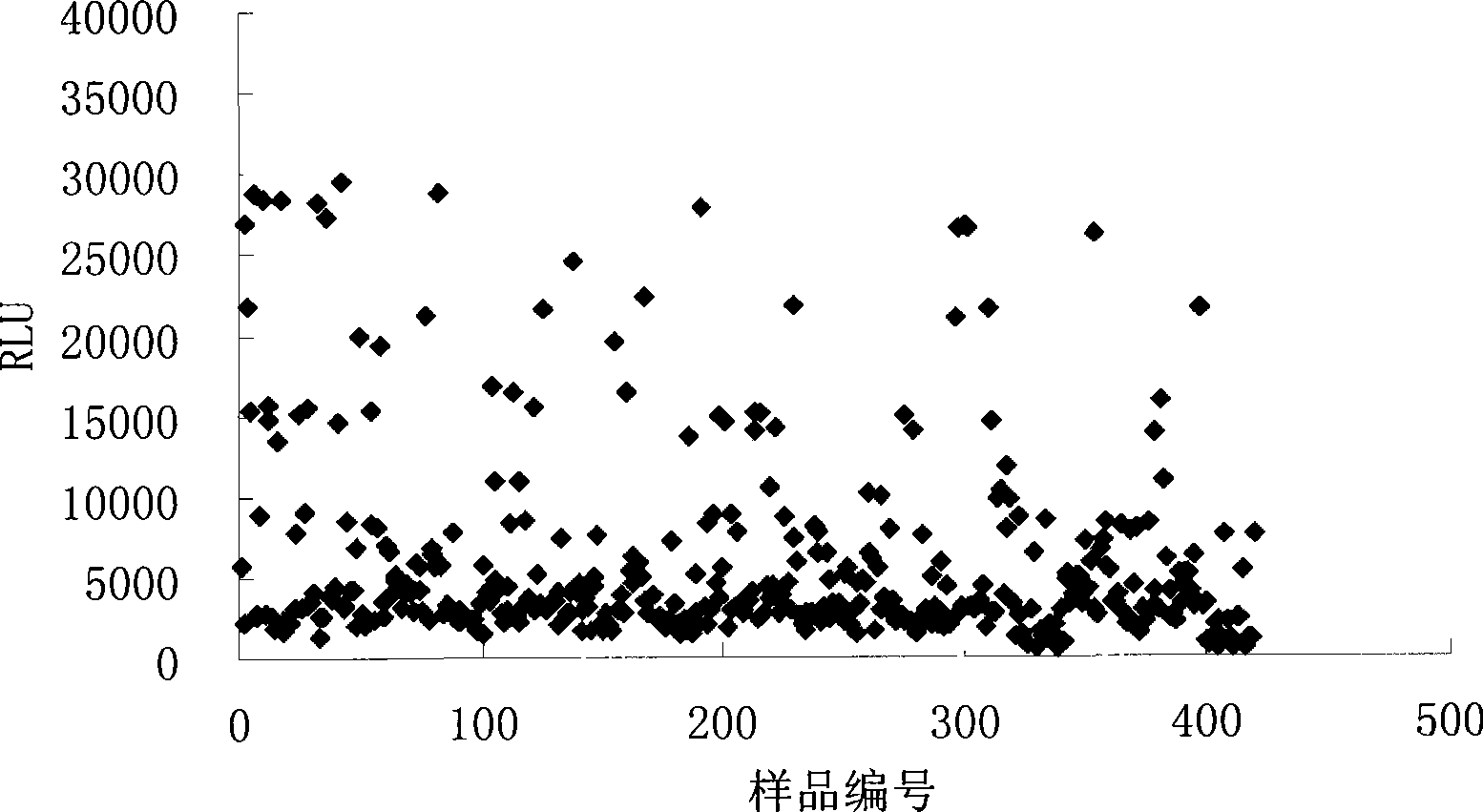
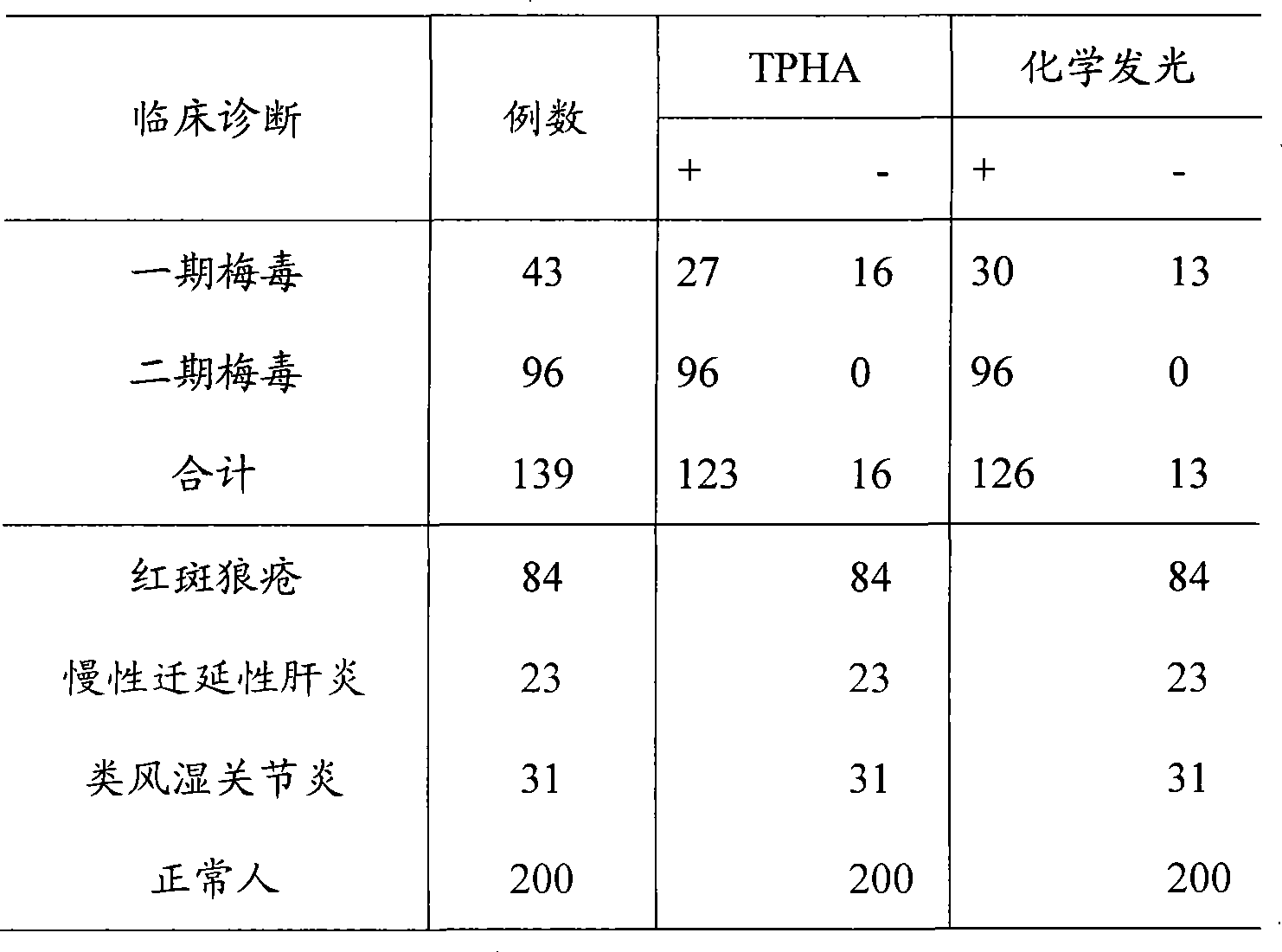
Syphilis helicoid antibody chemiluminescence immune assay determination kit and method for preparing same
A technology of chemiluminescent immunity and Treponema pallidum, which is applied in the field of immunoanalysis medical diagnosis, can solve the problems of inability to widely apply clinical diagnosis and scientific research work, and limit popularization and use, and achieve a shortened detection window period, non-infectious, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Treponema pallidum antibody chemiluminescence immunoassay assay kit of the present invention
[0039] 1. Preparation of enzyme-labeled antigen
[0040] Three Treponema pallidum specific proteins 15 (TpN15), 17 (TpN17) and 47 (TpN47) were labeled with sodium periodate oxidation labeling method and coupled with horseradish peroxidase. After adding 50% glycerol, it was aliquoted and stored at -20°C.
[0041] 2. Preparation of TP negative and positive control substances
[0042] Positive control serum: the high-titer positive human serum determined by the exact method is mixed into positive plasma (PositivePool, the number of plasma parts is greater than 5), heated and inactivated at 56°C for 1 hour, properly diluted with newborn bovine serum, and added with biological preservation Add 1 / 10000th (W / V) of food red to make it red, sterilize and filter, and store at 2-8°C.
[0043] Negative control serum: use human serum that is negative for syphili...
Embodiment 2~3
[0089] Embodiment 2~3 preparation TP assay kit of the present invention
[0090] Except that the TP-specific recombinant antigen was labeled with alkaline phosphatase, AMPPD was used as the chemiluminescent substrate, plastic beads were used as the carrier, the pH value of the coating solution was 8.4, and the pH value of the blocking solution was 7.5. The same method as Example 1 was used to prepare the TP assay kit.
Embodiment 4
[0091] Example 4 Preparation of TP assay kit of the present invention
[0092] The TP assay kit was prepared in the same manner as in Example 1 except that magnetic particles were used as the carrier.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com