Soluble pd-1 variants, fusion constructs, and uses thereof
A PD-1 and soluble technology, which is applied in the direction of fusion with soluble cell surface receptors, fusion polypeptides, and targeting specific cell fusion, can solve the problems of not improving Th1CD8T cell responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0154] Example 1 - Construction of Mouse sPD-1 Vaccine Candidates
[0155] This example illustrates the construction of a mouse sPD-1-p24 fusion construct. To construct the mouse sPD-1-p24-Fc construct, the PVAX vector carrying the wild-type msPD-1 gene and p24 gene was fused to rabbit Fc DNA. The vector and p24-Fc DNA were ligated by a linker encoding GGGSGGG (SEQ ID NO: 29). Transcription is under the control of the promoter Pcmv.
[0156] The mouse sPD1 protein variant mspd1-IgVΔ was obtained by deleting amino acids 89-90 of the mouse PD-1 protein, which form the C''D loop of the IgV domain and for PD-1 and PD-L1 / L2 interactions are essential. The PVAX vector carrying mspd1-IgVA was ligated to p24-rabbit Fc DNA via a linker encoding the GGGSGGG (SEQ ID NO:29) linker sequence. In addition, a p24-Fc fusion construct was obtained by ligating a p24-carrying PVAX vector with rabbit Fc DNA. Transcription is under the control of the promoter Pcmv.
[0157] Figure 1A An alig...
Embodiment 2
[0158] Embodiment 2—the binding ability of mouse SPD-1 fusion protein and SPD-1 ligand
[0159] This example shows the binding ability of msPD-1 fusion protein to mouse sPD-1 ligand. Briefly, 293T cells were transfected with PD-L (PD-L1 and PD-L2). The binding of sPD-1 protein to PD-1 ligand was detected by FITC-anti-rabbit Fc antibody using flow cytometry, and the results were analyzed by flowJo.
[0160] Such as Figure 2A The results shown in -B reveal that mspd1-p24-Fc binds to mouse PD-1 ligands PD-L1 and PD-L2. In contrast, the variant mspd1-IgVΔ fusion protein did not bind the mouse PD-1 ligand. Furthermore, p24-Fc did not affect the interaction between PD-1 and PD-L1 / L2.
Embodiment 3
[0161] Example 3 - Wild-type mouse SPD1 vaccine induces humoral and cell-mediated immune responses
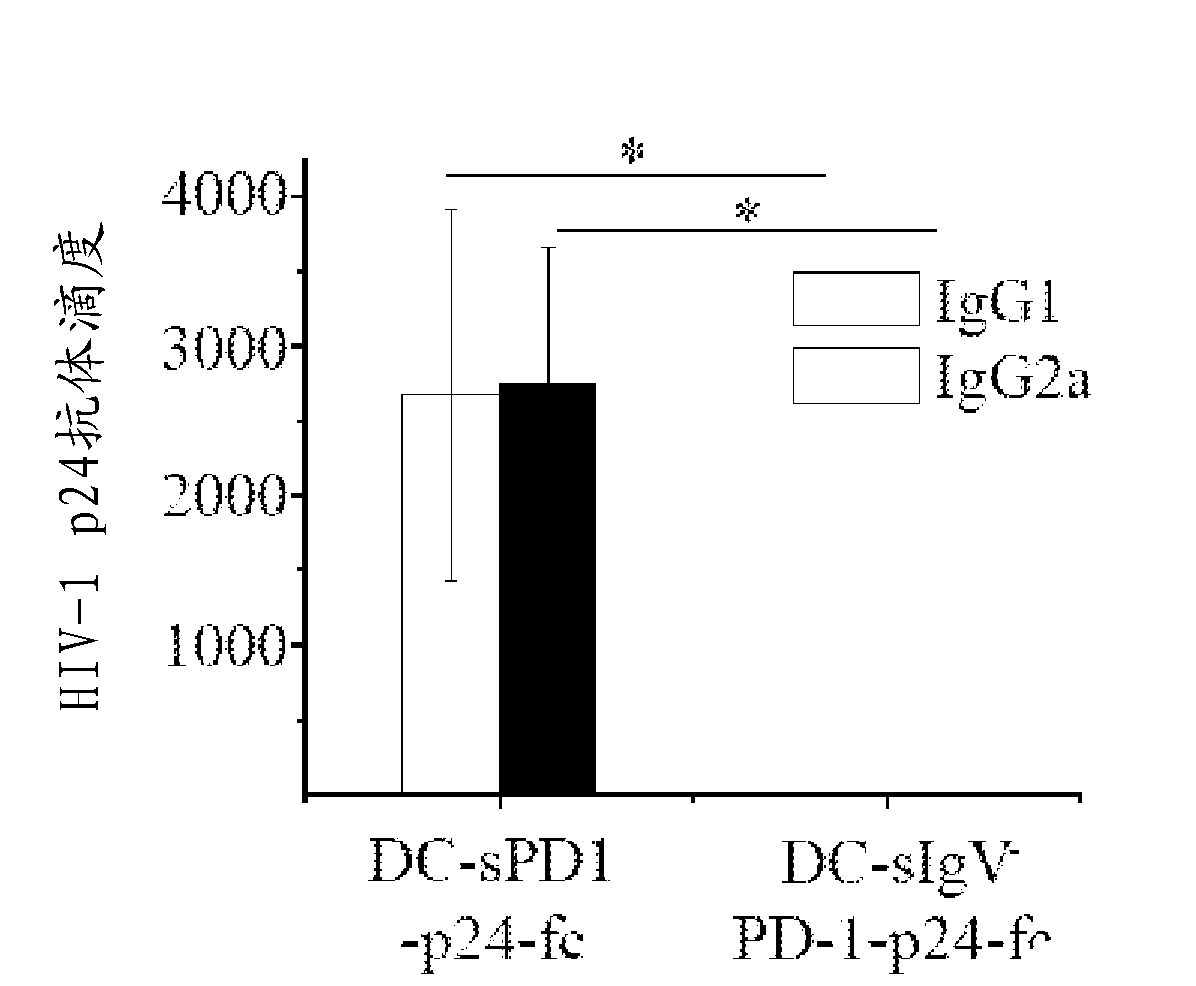
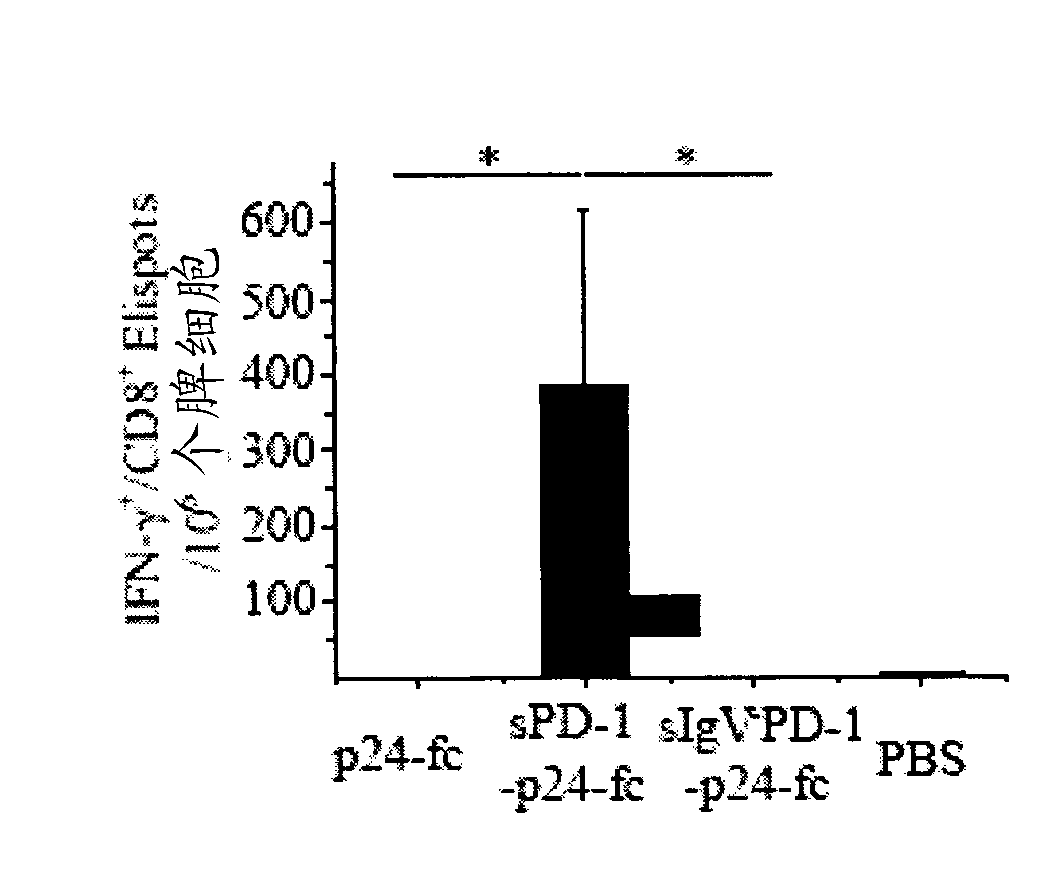
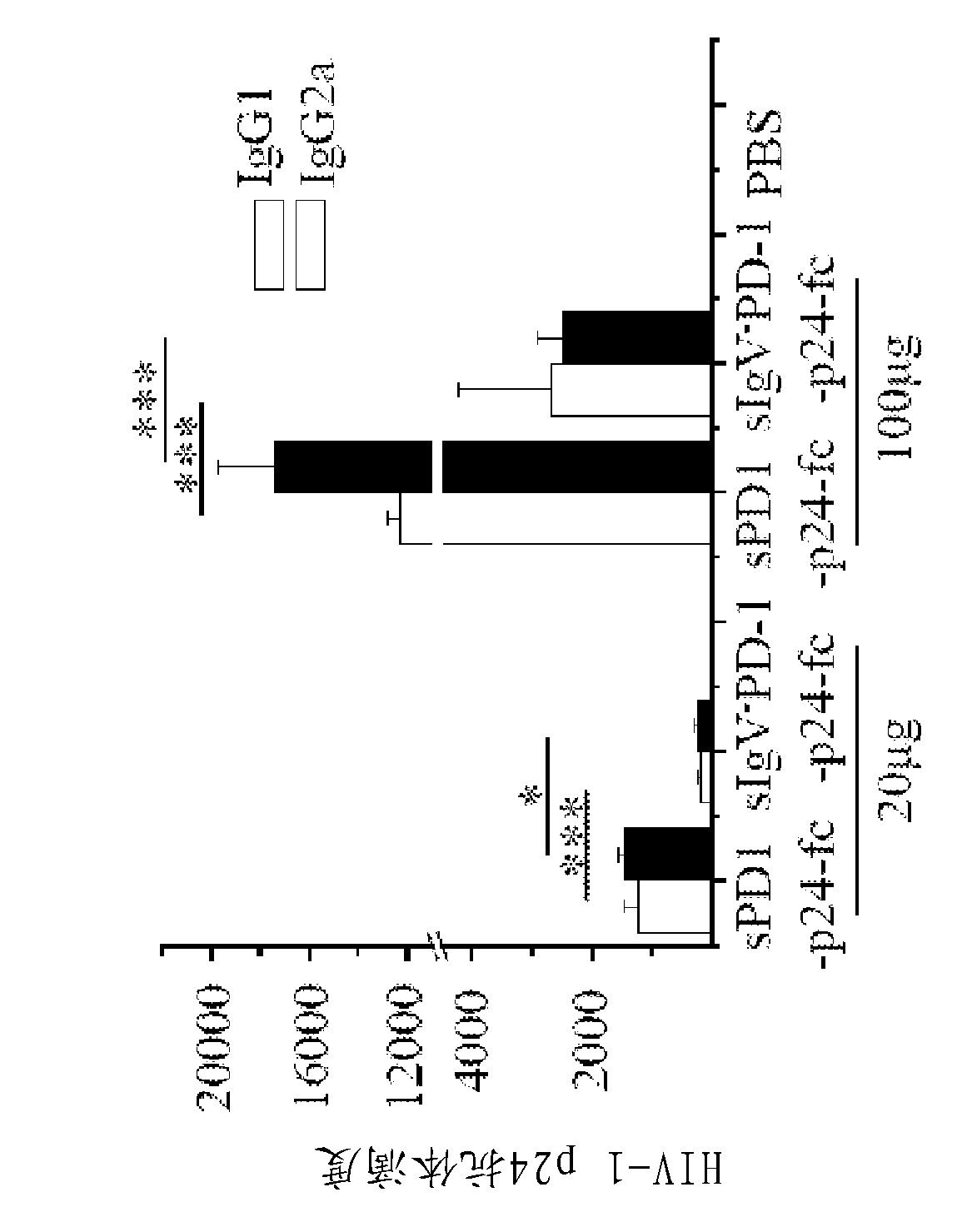
[0162] This example shows that wild-type msPDl-p24-Fc effectively induces both humoral and cell-mediated immune responses. Briefly, Balb / c mice were primed at week 0 and treated with 20 μg / mouse encoding msPd1-p24-Fc, mspd1-IgVA-p24-Fc, or p24-Fc at weeks 3 and 6. DNA vectors were enhanced via intramuscular electroporation. Mice receiving PBS served as controls.
[0163] Two weeks after the final immunization, mouse sera were collected and exposed to HIV-1 p24 viral protein. Levels of anti-p24 IgG1 and IgG2a antibodies were measured by ELISA. Anti-p24 antibody levels in control samples are not shown because absorbance readouts for these samples were below the cutoff value used to determine antibody titers. Anti-p24 antibody endpoint titers were defined as the reciprocal of the highest dilution of the test sample that produced a read at least twofold higher than that of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com