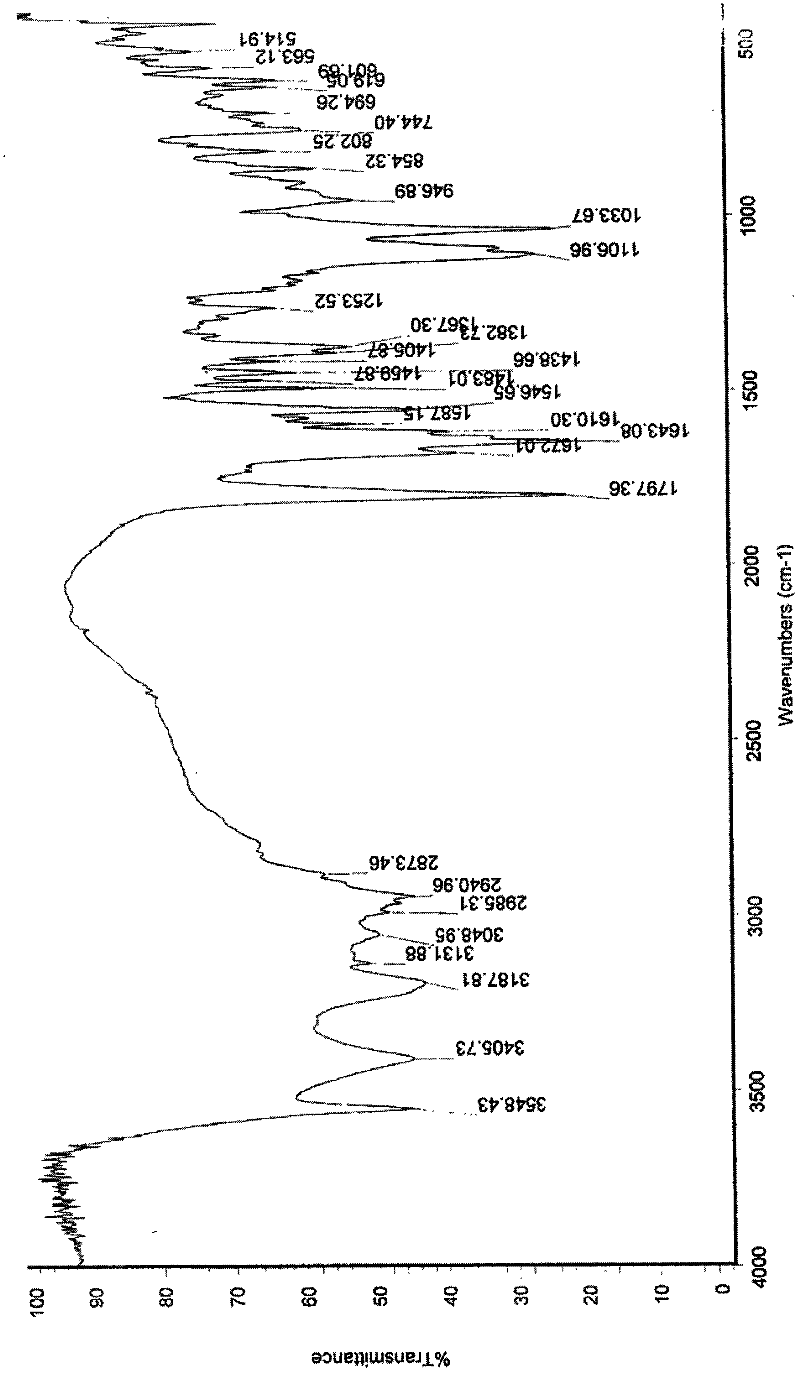
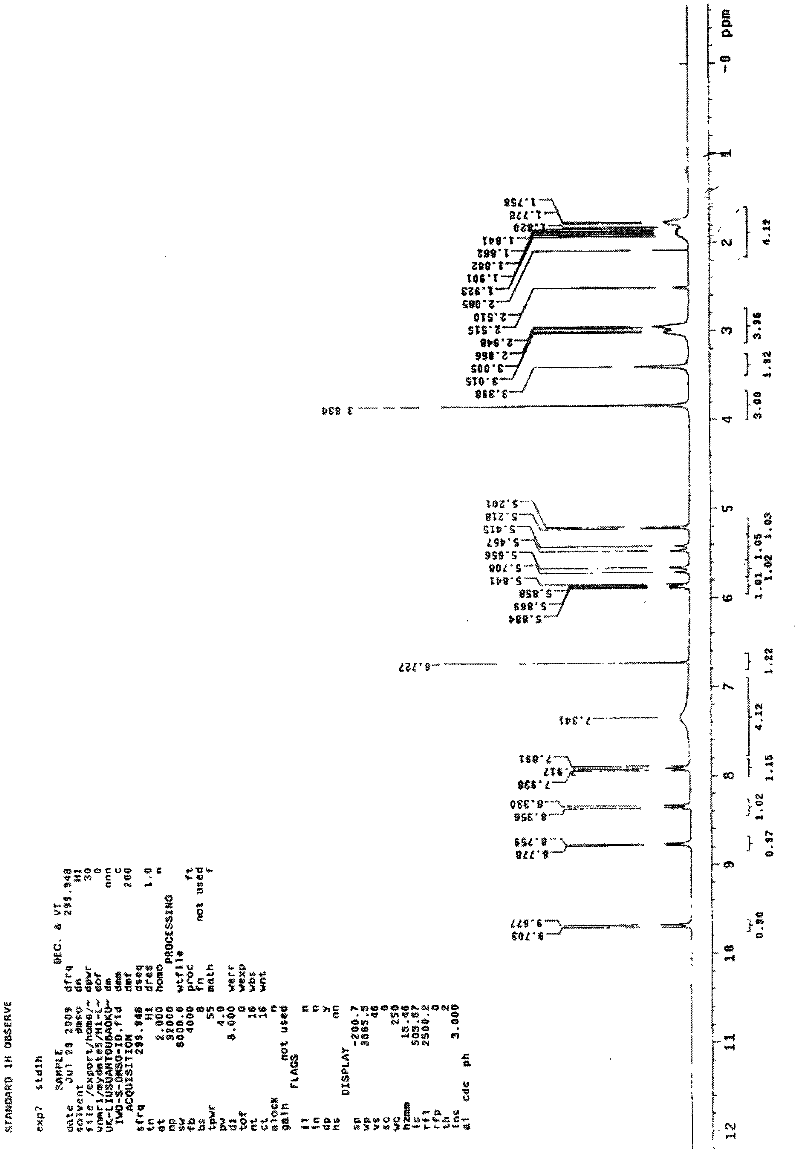
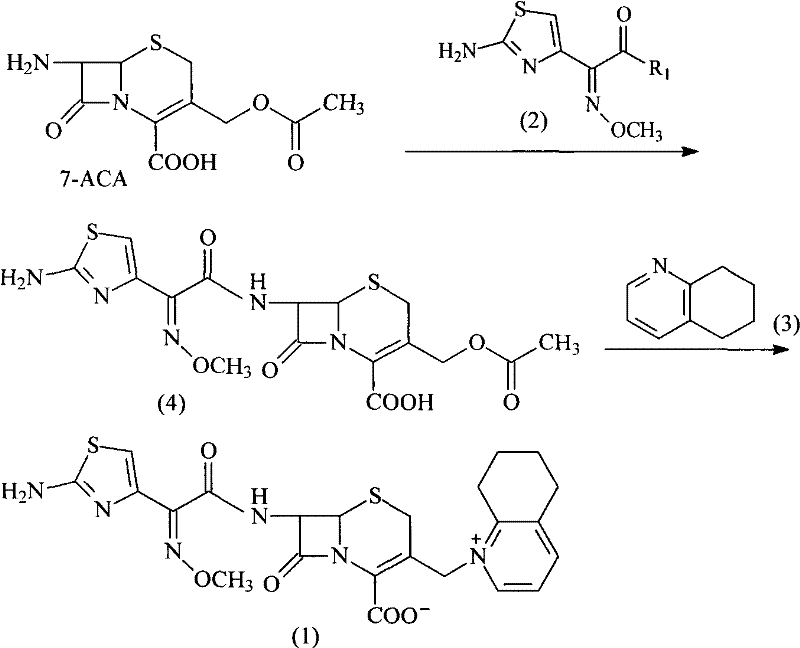
Synthetic method of cefquinome sulfate
A technique for the synthesis of cefquinome sulfate and its synthetic method, which is applied in the new synthesis process of antibiotic raw materials for animals, and in the field of synthesis of cefquinome sulfate, which can solve problems such as unsatisfactory product purity and color, harsh reaction conditions, and difficult separation and purification. , to achieve the effect of cheap raw and auxiliary materials, high product yield, and easy access to raw and auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] (1) Preparation of 7-phenylacetamido-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid p-methoxybenzyl halide (7)
[0036] 10 g of GCLE and 3.75 g of KI were added to 50 ml of dichloromethane, and the reaction was stirred at room temperature for 3-5 hours (reaction monitored by TLC). Cool down to below -10°C, add dropwise a mixture of 5,6,7,8-tetrahydroquinoline / dichloromethane 2.67g / 15ml, stir for 3 hours, add 80ml isopropyl ether dropwise, precipitate a solid, and filter with suction , washed, and dried in vacuo to obtain compound 11.05g with a yield of 92%, and the purity was detected by HPLC (≥80%).
[0037](2) Preparation of 7-amino-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid halide (8)
[0038] Add 10g of compound (7) (unrefined) to 50ml of dichloromethane, stir, cool to 0°C, add 2.1ml of pyridine dropwise, after the addition is complete, cool down to -10°C, add dropwise 5.6g of phosphorus pentachloride / 30ml Dichloromethane solution, ...
Embodiment 2
[0042] (1) Preparation of 7-phenylacetamido-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid p-methoxybenzyl halide (7)
[0043] 10 g of GCLE and 3.75 g of KI were added to 40 ml of acetone, and the reaction was stirred at room temperature for 3-5 hours (reaction monitored by TLC). Cool down to below -10°C, add dropwise a mixture of 5,6,7,8-tetrahydroquinoline / acetone 2.67g / 25ml, stir for 3 hours, add 80ml of isopropyl ether dropwise, precipitate a solid, suction filter, and wash , dried in vacuo to obtain compound 11.13g, the yield was 92.7%, and the purity was detected by HPLC (≥80%).
[0044] (2) Preparation of 7-amino-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid halide (8)
[0045] Add 10g of compound (7) (unrefined) to 50ml of dichloromethane, stir, cool to 0°C, add 3.27ml of triethylamine dropwise, after addition, cool down to -10°C, add 5.6g of phosphorus pentachloride dropwise / 50ml of dichloromethane solution, temperature control -10 ℃ fo...
Embodiment 3
[0067] (1) Preparation of 7-phenylacetamido-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid p-methoxybenzyl halide (7)
[0068] Add 10g of GCLE and 3.75g of KI to 40ml of dichloromethane, stir and react at room temperature for 3 to 5 hours (reaction monitored by TLC), cool down to below -10°C, add 5,6,7,8-tetrahydroquinone dropwise Phenyl / acetone 2.67g / 21ml (g:V) mixed solution was stirred for 3 hours, 80ml of isopropyl ether was added dropwise, a solid was precipitated, filtered with suction, washed, and dried in vacuo to obtain 11.38g of compound with a yield of 94.8%, detected by HPLC Purity (≥80%).
[0069] (2) Preparation of 7-amino-3-[(5,6,7,8-tetrahydroquinoline)methyl]-cephalosporanic acid halide (8)
[0070] Add 10g of compound (7) (unrefined) to 50ml of dichloromethane, stir, cool to 0°C, add 3.27ml of triethylamine dropwise, after addition, cool to -10°C, add dropwise 5.6g of phosphorus pentachloride / 45ml dichloromethane solution, and kept at -10°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com