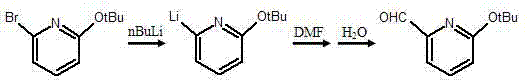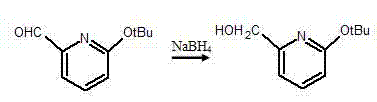Synthesis method of pyridine derivative 2-tert-butoxy-6-methylene chloropyridine
A technology of methylenechloropyridine and tert-butoxypyridine, which is applied in the field of synthesis of pyridine derivatives, can solve the problems of high reaction temperature, poisonous catalyst, and easy failure, and achieve high reactivity and few reaction steps , the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
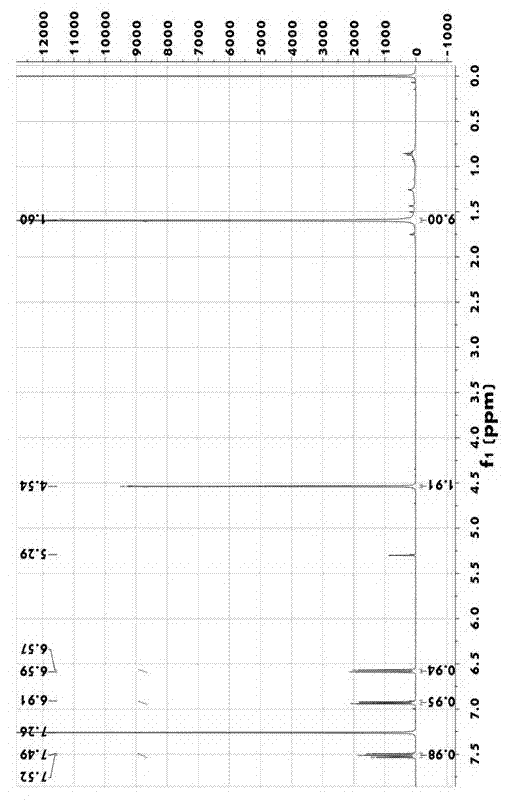
[0020] This example provides a synthetic method of pyridine derivative 2-tert-butoxy-6-methylenechloropyridine, the specific synthetic steps are as follows:
[0021] 1. In N 2 Under protection, add 7.0g (0.03mol) of 2-bromo-6-tert-butoxypyridine to the three-necked flask, add 100ml of toluene as a solvent, and add 12ml of n-butyllithium dropwise at -78°C (M=2.5mol / L) . After stirring at low temperature for 30 minutes, the system solution gradually changed from light yellow to orange yellow. At the above temperature, 4.4 g (0.06 mol) of N,N-dimethylformamide (DMF) was added and reacted at 0°C for 45 min, the color of the reaction solution system became light and yellow. After the reaction was completed, the liquid was extracted and separated with ethyl acetate. After drying and standing still, 6.0 g of the crude product 2-tert-butoxy-6-pyridinal was obtained by suspension evaporation, which was directly used for feeding in the next step. The reaction equation is as follo...
Embodiment 2
[0028] This example provides a synthetic method of pyridine derivative 2-tert-butoxy-6-methylenechloropyridine, the specific synthetic steps are as follows:
[0029] 1. In N 2 Under protection, add 7.0g (0.03mol) of 2-bromo-6-tert-butoxypyridine to the three-necked flask, add 100ml of toluene as a solvent, and add 12ml of n-butyllithium dropwise at -78°C (M=2.5mol / L) . After stirring at low temperature for 30 minutes, the system solution gradually changed from light yellow to orange yellow. At the above temperature, 4.4 g (0.06 mol) of N,N-dimethylformamide (DMF) was added and reacted at 0°C for 45 min, the color of the reaction solution system became light and yellow. After the reaction was completed, the liquid was extracted and separated with ethyl acetate. After drying and standing still, 6.0 g of the crude product 2-tert-butoxy-6-pyridinal was obtained by suspension evaporation, which was directly used for feeding in the next step. The reaction equation is as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com