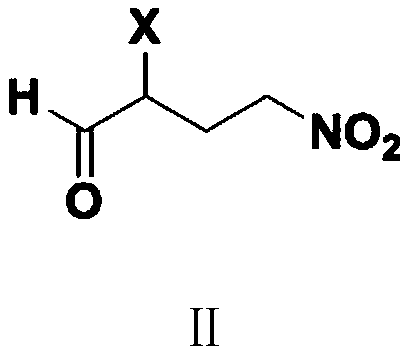
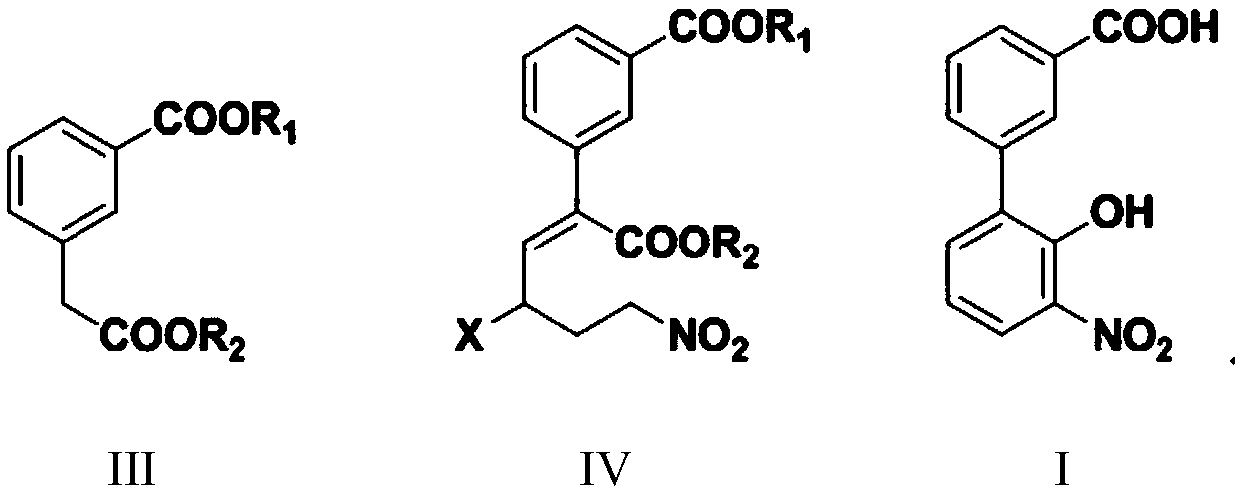
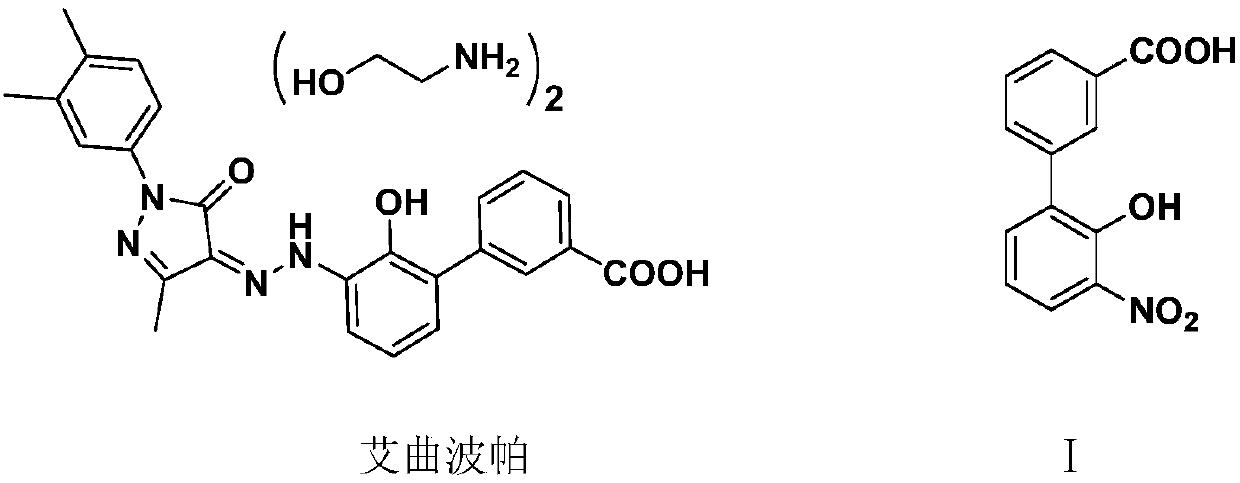
Preparation method of 3'-nitro-2'-hydroxybiphenyl-3-formic acid
A technology of hydroxybiphenyl and nitromethane, applied in the field of medicinal chemistry, can solve the problems of increasing the reaction cost and being unsuitable for large-scale production, and achieves the effects of low cost, high selectivity, and low production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: the preparation of 2-chloro-4-nitro n-butyraldehyde (Ⅱ1)
[0053] Add 30.0 g of THF, 9.0 g (0.1 mole) of 2-chloroacrolein, 6.2 g (0.1 mole) of nitromethane, and 0.1 g of DBU into a 500 ml four-necked flask connected with a stirring and thermometer, and stir at 45 to 50 °C React for 5 hours. After the solvent was recovered by distillation, the (80-100°C / 1-2mmHg) fraction was collected by vacuum distillation to obtain 13.9 g of 2-chloro-4-nitro-n-butyraldehyde (II1), with a yield of 91.8% and a gas phase purity of 99.7%.
Embodiment 2
[0054] Embodiment 2: the preparation of 2-chloro-4-nitro n-butyraldehyde (Ⅱ1)
[0055] Add 90.0 g (1.0 mol) of 2-chloroacrolein, 64.0 g (1.0 mol) of nitromethane, and 1.0 g of DBU into a 500 ml four-neck flask connected with a stirring and thermometer, and stir at 60 to 65° C. for 5 hours. Fractions (80-100°C / 1-2mmHg) were collected by distillation under reduced pressure to obtain 149.3 g of 2-chloro-4-nitro-n-butyraldehyde (II1), with a yield of 98.6% and a gas phase purity of 99.6%.
Embodiment 3
[0056] Embodiment 3: the preparation of 2-bromo-4-nitro n-butyraldehyde (Ⅱ2)
[0057] Add 30.0 g of acetonitrile, 13.5 g (0.1 mole) of 2-bromoacrolein, 6.2 g (0.1 mole) of nitromethane, and 0.1 g of DBU into a 500 ml four-neck flask connected with a stirring and thermometer, and stir at 55 to 60 ° C React for 3 hours. After recovering the solvent by distillation, the (95-115°C / 1-2mmHg) fraction was collected by vacuum distillation to obtain 17.6 g of 2-bromo-4-nitro-n-butyraldehyde (II2), with a yield of 89.8% and a gas phase purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com