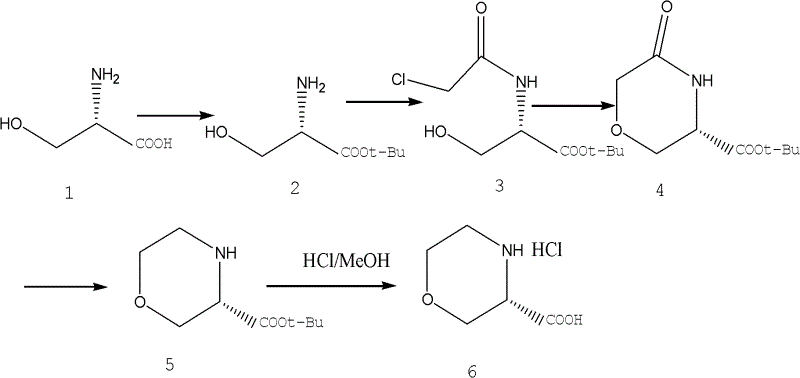
Novel synthetic method of (S)-3-morpholinyl carboxylic acid
A technology of morpholino carboxylic acid and a new method, which is applied in the synthesis of organic intermediates and pharmaceutical intermediates, can solve the problems of harsh reaction conditions, many by-products, and low yield, and achieve mild reaction conditions and few by-products , Response-specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The first step reaction operation process
[0031] Dissolve 10.5g of L-serine in 20ml of tert-butyl acetate, slowly add 2g of perchloric acid in 5ml of aqueous solution at 0°C, the reaction solution is slowly warmed to room temperature, stirred at room temperature for 8 hours, then washed with 10ml of water, 10ml Wash with ammonium chloride, combine the water phases, adjust the pH to 9-10 with potassium carbonate, extract with 100ml*3 dichloromethane, dry the dichloromethane with anhydrous sodium sulfate, and spin dry to obtain 10.0g (65.0%) of light yellow Oil intermediate 2 (L-serine tert-butyl ester).
[0032] The second step reaction operation process
[0033] Dissolve 10g of intermediate 2 in 100ml of dichloromethane, slowly add 8.4g of chloroacetic acid chlorine in 30ml of dichloromethane solution dropwise at 0°C, and slowly warm the reaction solution to room temperature, stir at room temperature for 1 hour, add 50ml (50 %) of sodium bicarbonate aqueous solution...
Embodiment 2
[0041] The first step reaction operation process
[0042] Dissolve 10.5g of L-serine in 30ml of tert-butyl acetate, slowly add 4g of perchloric acid in 5ml of aqueous solution at 0°C, and slowly heat up the reaction solution to 20-40°C, and stir at 20-40°C 10 hours, then washed with 15ml of water and 15ml of ammonium chloride, combined the water phases, adjusted the pH to 9-10 with potassium carbonate, extracted with 100ml*3 dichloromethane, dried the dichloromethane with anhydrous sodium sulfate, and spin-dried to obtain 10.0 g (65.0%) of intermediate 2 (tert-butyl L-serine) as a pale yellow oil.
[0043] The second step reaction operation process
[0044] Dissolve 10g of intermediate 2 in 100ml of dichloromethane, slowly add 13.5g of chloroacetic acid chlorine in 50ml of dichloromethane solution dropwise at 10°C, and slowly heat up the reaction solution to 20-30°C, and at 20-30°C Stir for 1 hour, add 50ml (50%) of aqueous sodium bicarbonate solution, separate layers, wash ...
Embodiment 3
[0052] The first step reaction operation process
[0053] Dissolve 10.5g of L-serine in 20ml of tert-butyl acetate, slowly add 3g of perchloric acid in 5ml of aqueous solution at 5°C, and slowly heat up the reaction solution to 50-60°C, and stir at 50-60°C for 8 hours , then washed with 10ml of water, washed with 10ml of ammonium chloride, combined the water phases, adjusted the pH to 9-10 with potassium carbonate, extracted with 100ml*3 dichloromethane, dried the dichloromethane with anhydrous sodium sulfate, and spin-dried to obtain 10.0g (65.0%) Intermediate 2 (tert-butyl L-serine) as pale yellow oil.
[0054] The second step reaction operation process
[0055] Dissolve 10g of intermediate 2 in 100ml of dichloromethane, slowly add dropwise 10g of chloroacetic acid chlorine in 30ml of dichloromethane solution at 0°C, the reaction solution is slowly heated to 30-40°C, and stirred at 30-40°C for 1 hour , add 50ml (50%) of aqueous sodium bicarbonate solution, separate layers, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com