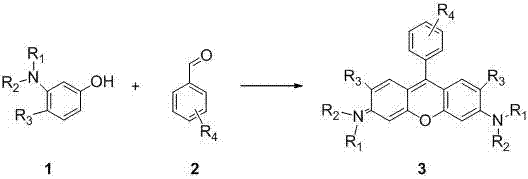
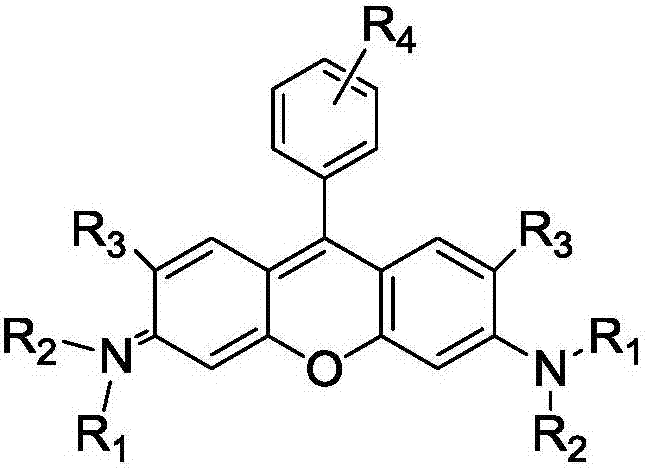
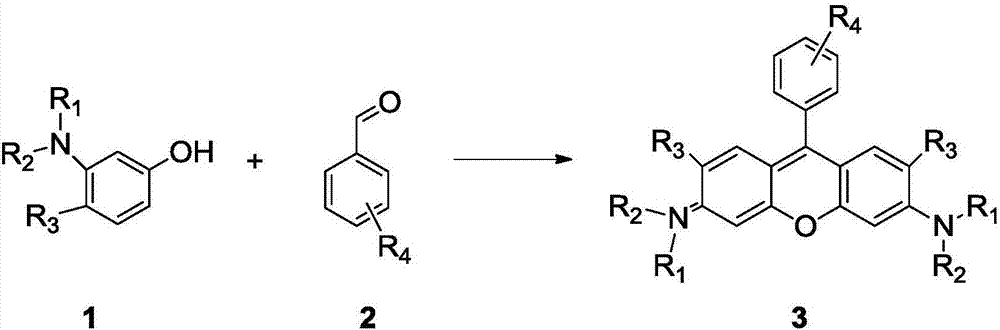
Method of rapidly preparing rhodamine dye with a plurality of active functional groups under mild condition
A technology of functional groups and conditions, applied in the direction of benzoxanthamine/xanthone/thioxanthone/selenoxanthone/tellurium xanthone dyes, organic chemistry, etc., can solve the price of functional rhodamine Expensive, difficult separation, application limitations and other problems, to achieve the effect of cheap catalyst, simple separation and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044] Put p-bromobenzaldehyde (1mmol, 185mg) and 3-hydroxy-N, N-diethylaniline (2mmol, 330mg) in a 25mL two-necked bottle, add p-toluenesulfonic acid (0.15mmol, 26mg), Add 15 mL of glacial acetic acid. Reaction at 70 degrees for 7 hours, at this time the solution turned dark purple, most of the acetic acid was removed under reduced pressure, and saturated NaHCO was added 3 , produce a lot of bubbles, until the purple precipitate is completely precipitated, filter out the purple precipitate, and dissolve it in 30mL of CH 2 Cl 2 Chlorobenzoquinone (0.5mmol, 122mg) was added to the solution, the color of the solution became darker, reacted for 2h, and CH was removed under reduced pressure 2 Cl 2 , separated on a silica gel column to obtain bright purple crystals. The yield was 45%. 1 H NMR (400MHz, CDCl 3 ):δ=7.79(d,2H,J=8.0Hz),7.32-7.28(m,4H),7.01-6.98(m,2H),6.86(s,2H),3.72-3.66(t,8H), 1.37-1.33ppm(m,12H). 13 C NMR (100MHz, CDCl 3 ): δ=158.1, 157.8, 131.2, ...
Embodiment 2
[0046]
[0047] Put m-bromobenzaldehyde (1mmol, 185mg) and -hydroxy-N, N-diethylaniline (2mmol, 330mg) in a 25mL two-neck flask, add p-toluenesulfonic acid (0.15mmol, 26mg), add 15 mL of glacial acetic acid. Reaction at 70 degrees for 7 hours, at this time the solution turned dark purple, most of the acetic acid was removed under reduced pressure, and saturated NaHCO was added 3 , produce a lot of bubbles, until the purple precipitate is completely precipitated, filter out the purple precipitate, and dissolve it in 30mL of CH 2 Cl 2 Chlorobenzoquinone (0.5mmol, 122mg) was added to the solution, the color of the solution became darker, reacted for 2h, and CH was removed under reduced pressure 2 Cl 2 , separated on a silica gel column to obtain bright purple crystals. The yield was 43%. 1 H NMR (400MHz, CDCl 3 ):δ=7.78(d,1H,J=7.8Hz),7.55-7.51(t,2H),7.36(d,1H,J=7.6Hz),7.30(s,1H),7.05(s,1H) ,6.97(d,2H,J=12.0Hz),6.86(s,2H),3.67-3.66(m,8H),1.35-1.31ppm(t,12H). 13 C NMR (1...
Embodiment 3
[0049]
[0050] Put p-fluorobenzaldehyde (1mmol, 124mg) and 3-hydroxy-N, N-diethylaniline (2mmol, 330mg) in a 25mL two-necked bottle, add p-toluenesulfonic acid (0.15mmol, 26mg), Add 15 mL of glacial acetic acid. Reaction at 70 degrees for 7 hours, at this time the solution turned dark purple, most of the acetic acid was removed under reduced pressure, and saturated NaHCO was added 3 , produce a lot of bubbles, until the purple precipitate is completely precipitated, filter out the purple precipitate, and dissolve it in 30mL of CH 2 Cl 2 Chlorobenzoquinone (0.5mmol, 122mg) was added to the solution, the color of the solution became darker, reacted for 2h, and CH was removed under reduced pressure 2 Cl 2 , separated on a silica gel column to obtain bright purple crystals with a yield of 41%. 1 H NMR (400MHz, CDCl 3 ):δ=7.38(d,2H,J=4.0Hz),7.33-7.31(m,4H),6.98(d,2H,J=8.0Hz),6.83(s,2H),3.67-3.65(m, 8H),1.34-1.23ppm(m,12H). 13 C NMR (100MHz, CDCl 3 ): δ=158.1, 155.7, 131...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com