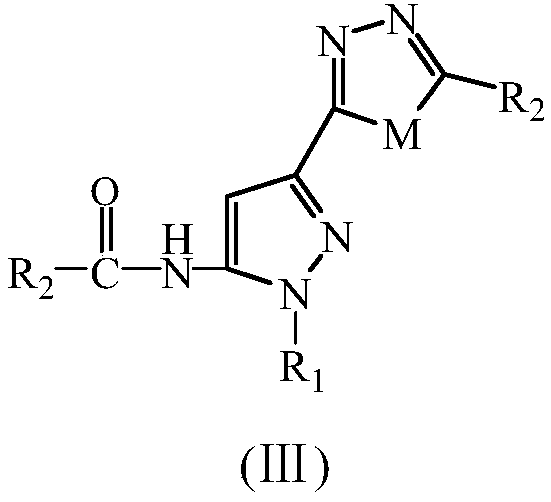
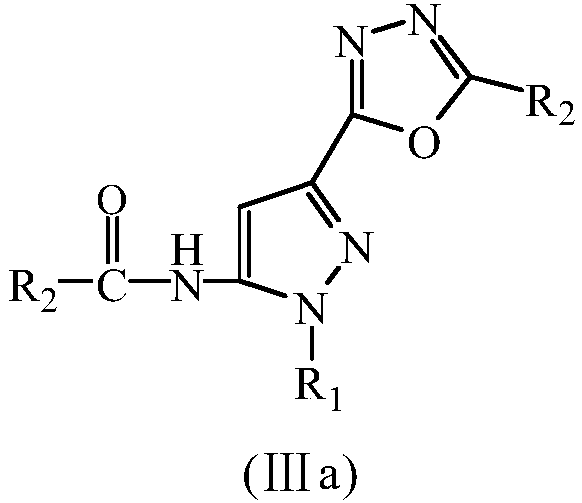
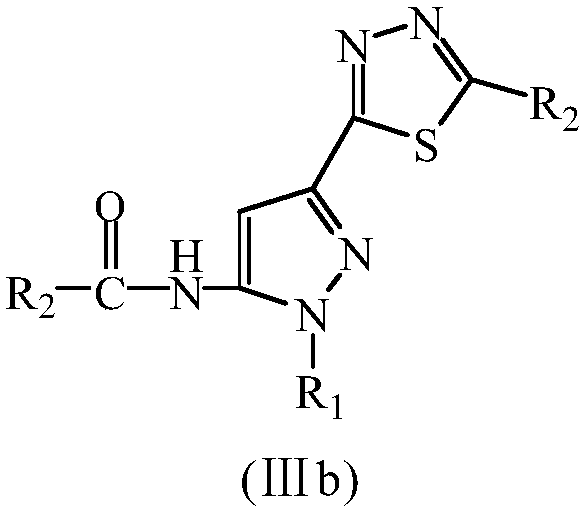
Diazole-pyrazolecarboxamide derivative and microwave hydrothermal synthesis method and application thereof
A kind of technology of derivatives and amides, applied in the application of such compounds, N--amide derivatives and the field of microwave hydrothermal synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1, N-[5-(5-methyl-[1,3,4]oxadiazol-2-yl)-2-cyclohexyl-2H-pyrazol-3-yl]-acetamide (Ⅲa 1 )Synthesis
[0069]
[0070] 5-Amino-1-cyclohexyl-1H-pyrazole-3-carbonitrile was synthesized from cyclohexylamine, 2,3-dicyano-propionic acid ethyl ester, ammonia water, etc. according to references: Chen Lianqing, Chen Guidi, Cheng Guosen, etc., 5 Synthesis and photophysical properties of -amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole derivatives[J].Journal of South Central University for Nationalities (Natural Science Edition), 2011,30(1); 5-12 self-made method.
[0071] (1) Take by weighing 15mmol 5-amino-1-cyclohexyl-1H-pyrazole-3-formonitrile with an analytical balance, add 30mL absolute ethanol thereto, shake and shake, then add 15mmol iron trichloride therein, Add a stirring magnet, stir and heat under reflux at 90°C for 14h, extract with ethyl acetate and water, and after rotary evaporation, perform silica gel column chromatography with ethyl acet...
Embodiment 2
[0075] Example 2, N-[5-(5-ethyl-[1,3,4]oxadiazol-2-yl)-2phenyl-2H-pyrazol-3-yl]-propionamide (Ⅲa 2 )Synthesis
[0076]
[0077] 5-Amino-1-phenyl-1H-pyrazole-3-carbonitrile was synthesized from aniline, 2,3-dicyano-propionic acid ethyl ester, ammonia water, etc. According to references: Chen Lianqing, Chen Guidi, Cheng Guosen, etc., 5-amino Synthesis and photophysical properties of -3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole derivatives[J].Journal of South Central University for Nationalities (Natural Science Edition),2011, 30(1); the method of 5-12 is self-made.
[0078] (1) Take by weighing 15mmol 5-amino-1-phenyl-1H-pyrazole-3-formonitrile with an analytical balance, add 30mL absolute ethanol thereto, shake and shake well, then add 15mmol iron trichloride therein, Add a stirring magnet, stir and heat under reflux at 90°C for 15 hours, then extract with ethyl acetate and water, and after rotary evaporation, use ethyl acetate and petroleum ether with a volum...
Embodiment 3
[0082] Example 3, N-[2-(4-hydroxy-phenyl-5-(5-propyl-[1,3,4]oxadiazol-2-yl)-2H-pyrazol-3-yl] -Butanamide (Ⅲa 3 )Synthesis
[0083]
[0084] 5-Amino-1-(4-hydroxy-phenyl)-1H-pyrazole-3-carbonitrile was synthesized from p-hydroxyaniline, 2,3-dicyano-propionic acid ethyl ester, ammonia water, etc. According to the reference: Chen Lianqing, Chen Guidi, Cheng Guosen et al. Synthesis and photophysical properties of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole derivatives[J].Journal of South Central University for Nationalities (Natural Science Edition), 2011, 30(1); self-made by the method of 5-12.
[0085] (1) Weigh 15mmol of 5-amino-1-phenyl-1H-pyrazole-3-carbonitrile with an analytical balance, add 30mL of n-propanol, shake and shake well, then add 15mmol of ferric chloride to it, add and stir Magneto, under stirring, heating at 85°C for 16h under reflux, then extracting with ethyl acetate and water, after rotary evaporation, use ethyl acetate and petrole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com