Preparation method of cefquinome sulfate
A technology of cefquinome sulfate and cefquine hydroiodide, which is applied in the field of preparation of cefquinome sulfate, can solve the problems of low total yield, unstable product color and the like, and achieves increased solubility, easy deprotection and mild conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
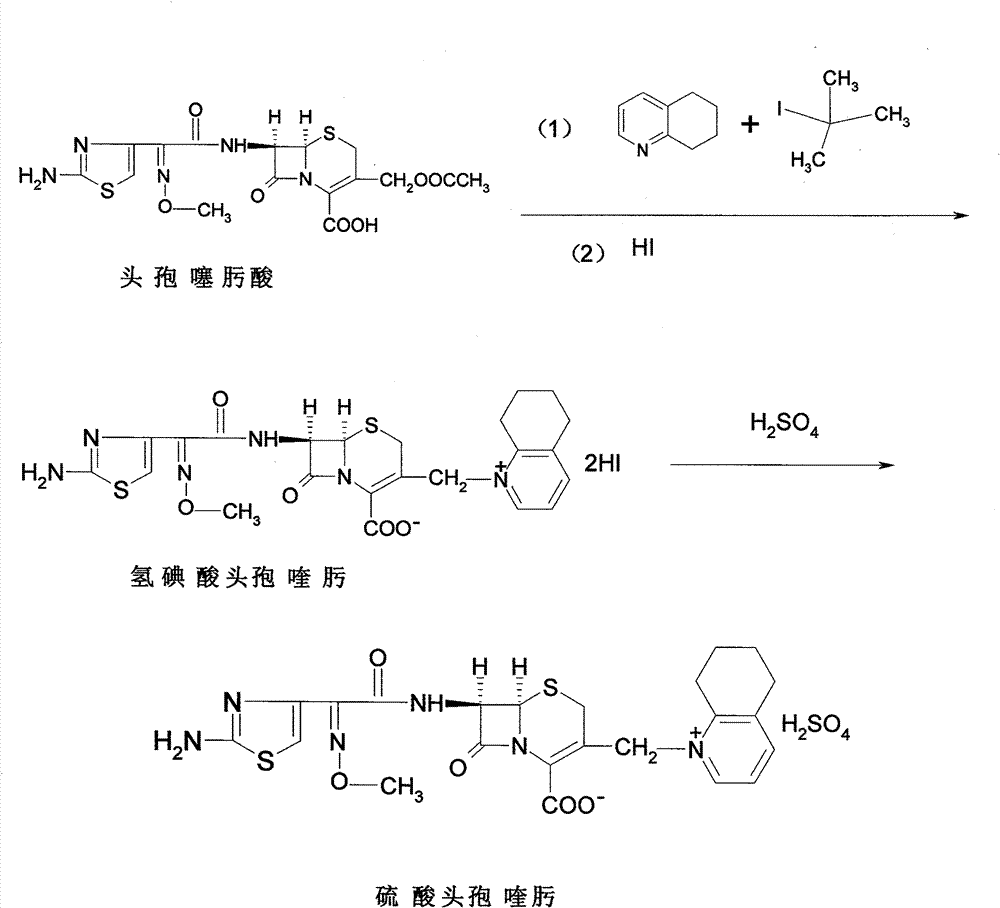
[0028] 1) preparation of cefquinome hydroiodic acid
[0029] Add 1300g of dichloromethane, 150g of iodotrimethylsilane (equivalent to 0.77mol), 114g of 2,3-cyclohexylpyridine (equivalent to 0.75mol), and 46g of cefotaxime (equivalent to 0.1mol) in a 3000ml three-necked flask in sequence , warming up to reflux, sampling once every half an hour (silica gel GF254 thin-layer plate monitors the conversion of cefotaxime, using ethyl acetate: methanol: water = 1: 4: 1 as developing agent) until the reaction of cefotaxime is complete, and the reflux ends , cooled to -4~-6°C in an ice-salt bath, dropwise added hydroiodic acid (a mixture of 150g potassium iodide and 650ml 2M hydrochloric acid, prepared in advance), and kept the temperature below 0°C, and the dropwise addition was completed in about 20~30 minutes. HPLC detection conversion rate ≥ 90%. Keep frozen for 6 hours, fully crystallize, filter with suction, wash and dry.
[0030] 2) preparation of cefquinome sulfate
[0031] A...
Embodiment 2
[0033] 1) preparation of cefquinome hydroiodic acid
[0034] Add 3000g of dichloromethane, 404g of iodotrimethylsilane (equivalent to 2.0mol), 297g of 2,3-cyclohexylpyridine (equivalent to 2.0mol), and 92g of cefotaxime (equivalent to 0.2mol) in a 5000ml three-necked flask. , warming up to reflux, sampling once every half an hour (silica gel GF254 thin-layer plate monitors the conversion of cefotaxime, using ethyl acetate: methanol: water = 1: 4: 1 as developing agent) until the reaction of cefotaxime is complete, and the reflux ends , cooled to -4~-6°C in an ice-salt bath, dropwise added hydroiodic acid (a mixture of 150g potassium iodide and 650ml 2M hydrochloric acid, prepared in advance), and kept the temperature below 0°C, and the dropwise addition was completed in about 20~30 minutes. HPLC detection conversion rate ≥ 90%. Keep frozen for 6 hours, fully crystallize, filter with suction, wash and dry.
[0035] 2) preparation of cefquinome sulfate
[0036] Add 800g of to...
Embodiment 3
[0038] 1) preparation of cefquinome hydroiodic acid
[0039] Add 700 g of dichloromethane, 40 g of iodotrimethylsilane (equivalent to 0.2 mol), 30 g of 2,3-cyclohexylpyridine (equivalent to 0.2 mol), and 46 g of cefotaxime (equivalent to 0.1 mol) into a 3000 ml three-necked flask in sequence , warming up to reflux, sampling once every half an hour (silica gel GF254 thin-layer plate monitors the conversion of cefotaxime, using ethyl acetate: methanol: water = 1: 4: 1 as developing agent) until the reaction of cefotaxime is complete, and the reflux ends , cooled to -4~-6°C in an ice-salt bath, dropwise added hydroiodic acid (a mixture of 150g potassium iodide and 650ml 2M hydrochloric acid, prepared in advance), and kept the temperature below 0°C, and the dropwise addition was completed in about 20~30 minutes. HPLC detection conversion rate ≥ 90%. Keep frozen for 6 hours, fully crystallize, filter with suction, wash and dry.
[0040] 2) preparation of cefquinome sulfate
[0041...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com