Monodisperse nano cefquinome sulfate liposome preparation and preparation method thereof
A kind of technology of cefquinoxime sulfate and liposome preparation, which is applied in the field of monodisperse nanometer cefquinoxime sulfate liposome preparation and preparation thereof, and can solve the problem of difficulty in guaranteeing insoluble particles, poor stability and uniformity, inability to meet quality requirements, etc. problem, to achieve the effect of improving drug availability, good stability and uniform size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
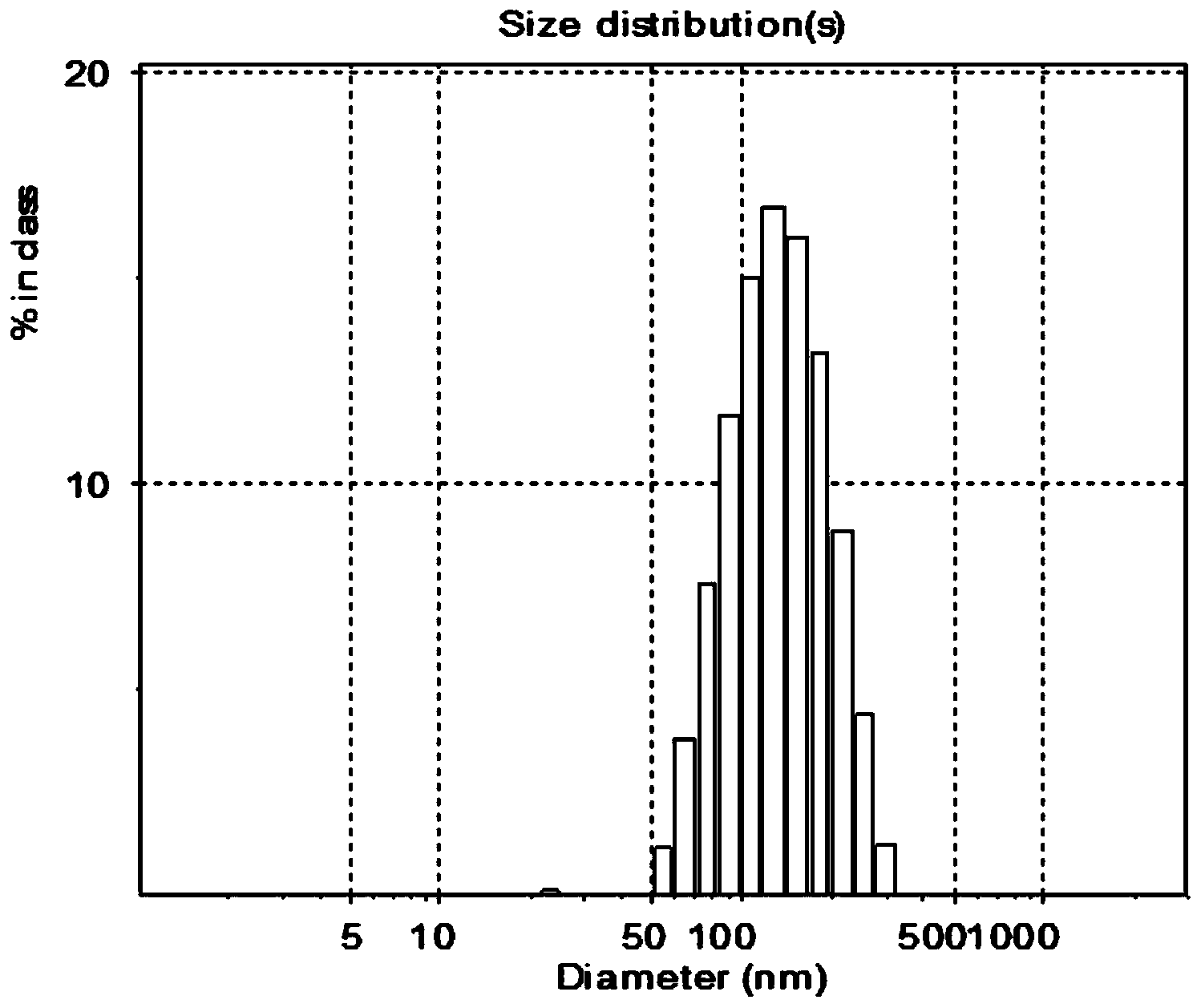
Image
Examples
Embodiment 1
[0032] formula:
[0033]
[0034] Preparation Process:
[0035] 1) Preparation of continuous phase: add sterile cefquinome sulfate to the reactor at room temperature and suspend it in water for injection; add sterile L-lysine to adjust the pH value to make it completely dissolved to obtain cefquinome sulfate / lysine Aqueous acid solution. After adding, adjust the pH value to 5.6; wherein the weight of aseptic cefquinoxime sulfate is C 23 H 24 N 6 O 5 S 2 ·H 2 SO 4 ·H 2 O meter.
[0036] 2) Preparation of disperse phase: dissolving phospholipid for injection, cholesterol and antioxidant in ethanol in the reactor, and stirring to obtain disperse phase solution;
[0037] 3) Preparation of monodisperse nano cefquinoxime sulfate liposome preparation: The dispersed phase is uniformly injected into the continuous phase through the porous membrane under the action of pressure (0.1 kPa), and the ethanol is removed under reduced pressure. Filtration through a microporous me...
Embodiment 2
[0039] formula:
[0040]
[0041] Preparation Process:
[0042] 1) Preparation of continuous phase: add sterile cefquinome sulfate to the reactor at room temperature and suspend it in water for injection; add sterile L-lysine to adjust the pH value to make it completely dissolved to obtain cefquinome sulfate / lysine Aqueous solution of amino acid; after adding, adjust pH value to 6.1; wherein the weight of aseptic β-cefaquime sulfate is C 23 H 24 N 6 O 5 S 2 ·H 2 SO 4 ·H 2 O meter.
[0043] 2) Preparation of dispersed phase: dissolving phospholipids for injection, cholesterol and vitamin E in ethanol in a reactor to obtain a dispersed phase;
[0044] 3) Preparation of monodisperse nano cefquinoxime sulfate liposome preparation: the dispersed phase is uniformly injected into the continuous phase through the porous membrane under pressure, the ethanol is removed under reduced pressure, and then filtered with a 0.45 μm microporous membrane , finally filtered through a...
Embodiment 3
[0046] formula:
[0047]
[0048] Preparation Process:
[0049] 1) Preparation of continuous phase: add sterile cefquinome sulfate to the reactor at room temperature and suspend it in water for injection; add sterile L-lysine to adjust the pH value to make it completely dissolved to obtain cefquinome sulfate / lysine The aqueous solution of amino acid; After adding, adjust pH value 6.5; Wherein the weight of aseptic cefquinoxime sulfate is C 23 H 24 N 6 O 5 S 2 ·H 2 SO 4 ·H 2 O meter.
[0050] 2) Preparation of dispersed phase: dissolving phospholipid for injection, cholesterol and antioxidant in ethanol in a reactor to obtain a dispersed phase;
[0051] 3) Preparation of monodisperse nano cefquinoxime sulfate liposome preparation: the dispersed phase is uniformly injected into the continuous phase through the porous membrane under pressure, the ethanol is removed under reduced pressure, and then filtered with a 0.45 μm microporous membrane , finally filtered throug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com