Cefquinome-sulfate breast injection agent for dry period of dairy cows and preparation method thereof
A technology of cefquinome sulfate and breast infusion agent, applied in the field of cefquinome sulfate breast infusion agent and preparation thereof, can solve the problems of long stay time of antibiotics, reduced incidence of mastitis, and reduced scope of influence, and achieves drug delivery. Convenient and fast, easy to scale up production, low local tissue irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Cefquinome sulfate breast injection (dry milk stage) provided by the invention is a kind of uniformly distributed preparation, and concrete preparation method comprises the following steps:
[0037] A. Let the vegetable oil (such as neutral soybean oil) cool after filtration and heat sterilization;
[0038] B. Take an appropriate amount of pretreated vegetable oil and add 0.03-0.3g of glyceryl monostearate;
[0039] C. Heat at 60-80°C for 15-30 minutes, wait for the glyceryl stearate to melt, stir evenly, cool to about 40°C, add 0.03-0.3g suspending agent (such as Span 80), 0.05-0.3 g (in terms of cefquinome) of cefquinome sulfate (5-10 μm in particle size), add vegetable oil to a total amount of 3.0ml;
[0040] D. grind evenly, make cefquinome sulfate breast injection;
[0041] F. Put the medicine into the injection tube, seal it, sterilize it, inspect it with light, and pack it.
[0042] In the following examples, the detection method of the sedimentation particle ...
Embodiment 1
[0045] Embodiment 1, the prescription screening of cefquinome sulfate breast injection (dry period)
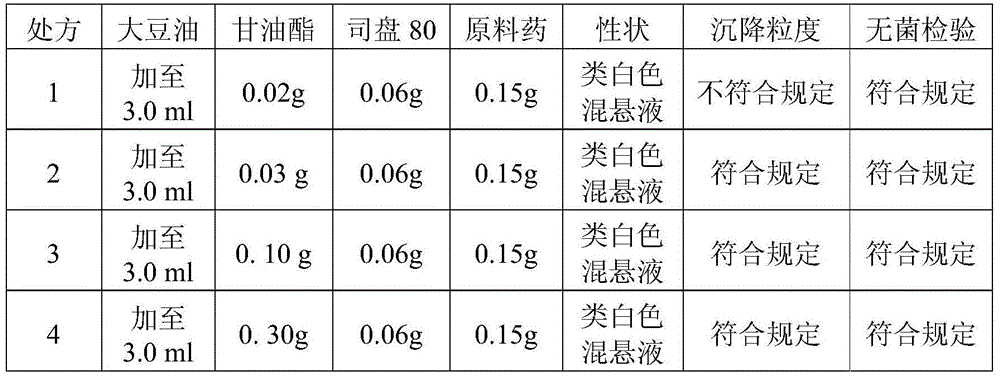
[0046] 1. Screening of glyceryl monostearate content in the formula (note: glyceryl monostearate in the table is referred to as glyceride)
[0047] Table 1
[0048]
[0049] The preparation method is as follows:
[0050] 1) Cefquinoxime sulfate aseptic powder is obtained through ultrafine pulverization to obtain cefquinoxime sulfate aseptic micropowder (particle diameter 5-10 μm);
[0051] 2) After the soybean oil is filtered and heat sterilized (sterilized at 150° C. for 3 hours), it is placed at room temperature for use;
[0052] 3) Take an appropriate amount of pretreated soybean oil, add glyceryl stearate and suspending agent Span 80; heat and stir (heating at 80°C for 15 minutes), after the glyceryl stearate melts, add cefquinone sulfate Oxime;
[0053] 5) Grind evenly in a sterile colloid mill to prepare cefquinome sulfate breast injection.
[0054] As can be see...
Embodiment 2
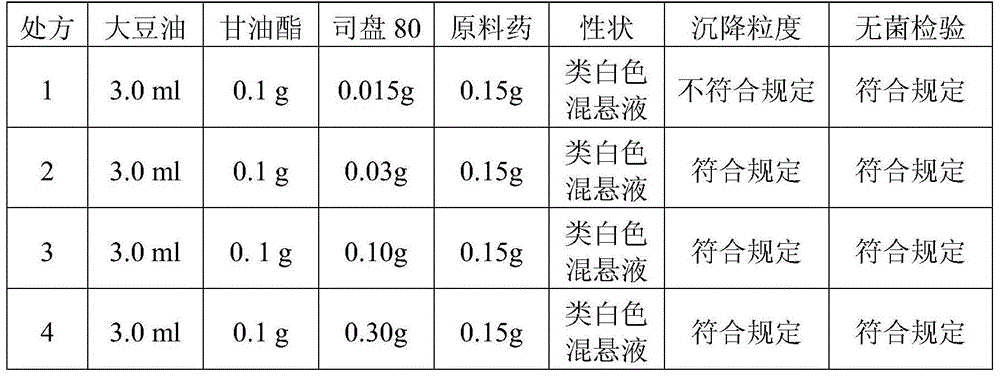
[0060] Embodiment 2, preparation cefquinome sulfate breast injection (dry period)
[0061]
[0062] The preparation method is as follows:
[0063] 1) the aseptic powder of cefquinoxime sulfate is ultrafinely pulverized to obtain the aseptic micropowder of cefquinoxime sulfate with a particle size of 5-10 μm;
[0064] 2) After the soybean oil is filtered and heat sterilized (sterilized at 150° C. for 3 hours), it is placed at room temperature for use;
[0065] 3) Take an appropriate amount of pretreated soybean oil (40%-60% of the total amount of soybean oil), add glyceryl monostearate; heat and stir (heating at 80°C for 15 minutes), wait for glyceryl monostearate Melt the ester, stir evenly, cool to 40°C, add Span 80, cefquinome sulfate, and add soybean oil to the full amount;
[0066] 4) Grind evenly in a sterile colloid mill to prepare cefquinome sulfate breast injection.
[0067] The properties of the obtained cefquinome sulfate breast injection (dry milk stage) are off...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com