Preparation method and application of cefquinome sulfate sustained-release suspension injection
A technology of cefquinome sulfate and suspension injection, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, liquid delivery, etc., to achieve stable quality and reliable curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of preparation method of cefquinome sulfate slow-release suspension injection, select optimal formula by optimal formula and orthogonal test, and use colloid mill to grind through, make injection reach suspension sustained-release effect; Concrete steps are as follows:
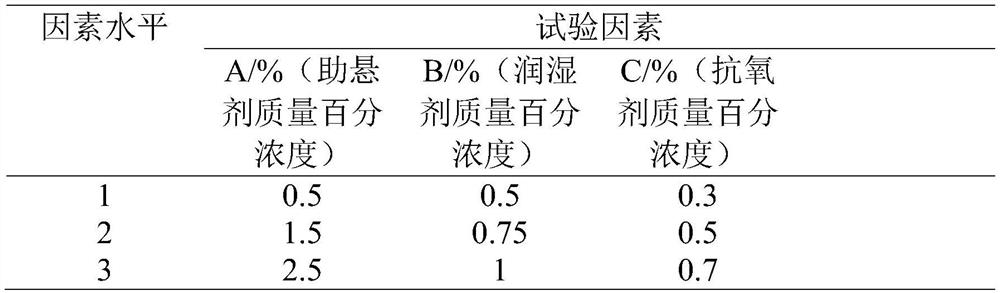
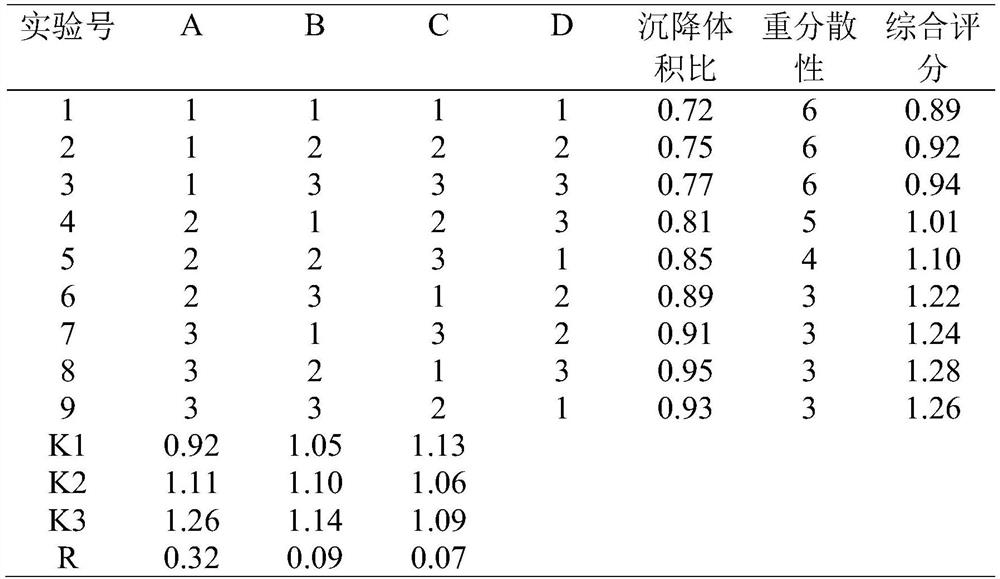
[0024] 1) Through screening experiment, the present invention takes ethyl oleate for injection as dispersion medium, hydrogenated castor oil is suspending agent, Span 85 is wetting agent, butylated hydroxytoluene (BHT) is antioxidant and uses normal Cross test optimization; And draw by orthogonal test analysis, the concentration of hydrogenated castor oil is 2.5%, the Span 60 concentration is 1%, and the BHT concentration is 0.3% to be optimal formula (as shown in table 1 and table 2);
[0025] 2) Add an appropriate amount of ethyl oleate for injection according to the N 2 Heating to 120°C under protection, and keeping warm for 2 hours;
[0026] 3) When the liquid is cooled to 80°C, add BHT, hyd...
Embodiment 2
[0033] An application of cefquinome sulfate sustained-release suspension injection, 12 SD rats were randomly divided into two groups of 6 rats in each group, half male and half male, six using the injection without improved technology, and six using the improved Injection solution after rubber milling (as shown in Table 3), administration method: intramuscular injection, administration dosage: 0.18mL / kg. Blood collection time: 5min, 15min, 30min, 45min, 1h, 1.5h, 2h, 3h, 4h, 6h, 8h, 12h, 24h, 48h, a total of 14 blood collection points per SD rat. Table 4 shows the post-treatment process of blood collection.
[0034]
[0035] table 3
[0036] Area=Area 134.1 / α
[0037] Note: Area 134.1 To detect the quantitative ion pair (529 / 134.1) peak area. α is the ratio of the L6 peak area of the quality control solution and the linear solution in the detection of each group of rats.
[0038] Blood drug mass percent concentration (peak area>QC) C=(Area+3779.3776) / 147.6353
[0...
Embodiment 3
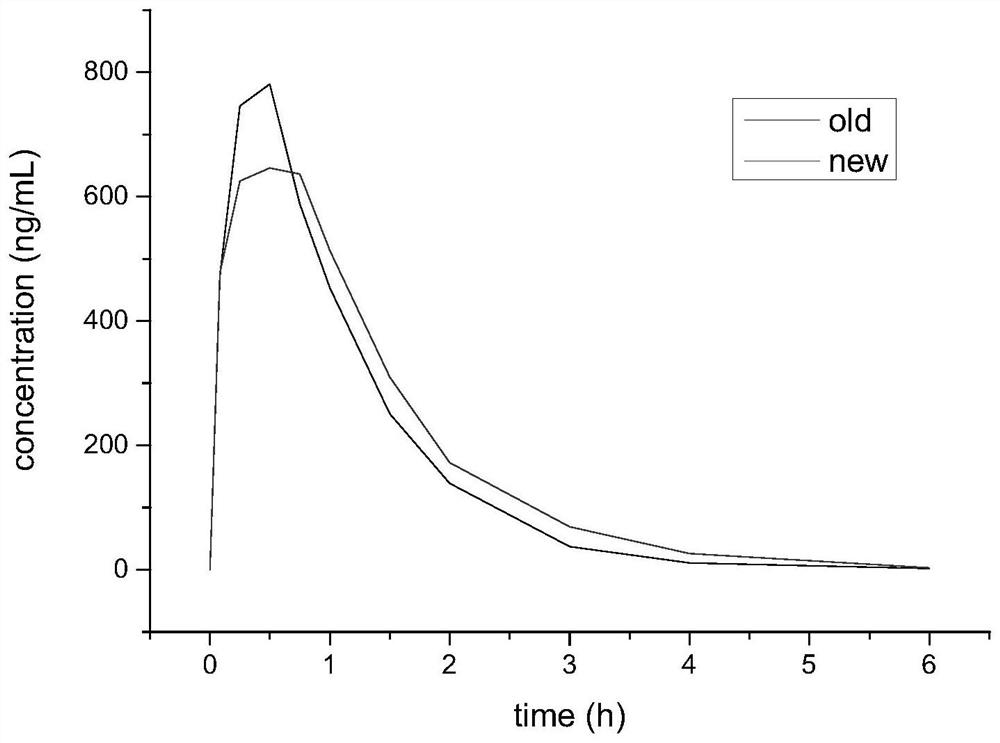
[0044] A kind of ultra high performance liquid chromatography-tandem mass spectrometry (UPLC-MS / MS measures the mass percentage concentration of cefquinome sulfate in SD rat plasma, draws cefquinome sulfate drug-time curve (such as figure 1 shown), to calculate the relative bioavailability.
[0045]The present invention selects the best formula by optimizing the formula and orthogonal test, and uses the colloid mill to grind, and the cefquinome sulfate injection of the new technology is compared with the cephalosporin sulfate injection of the old technology, within 0 to 6 hours. , The new technology has the effect of slow absorption and slow release compared with the old technology preparation, and the sustained release effect is prolonged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com