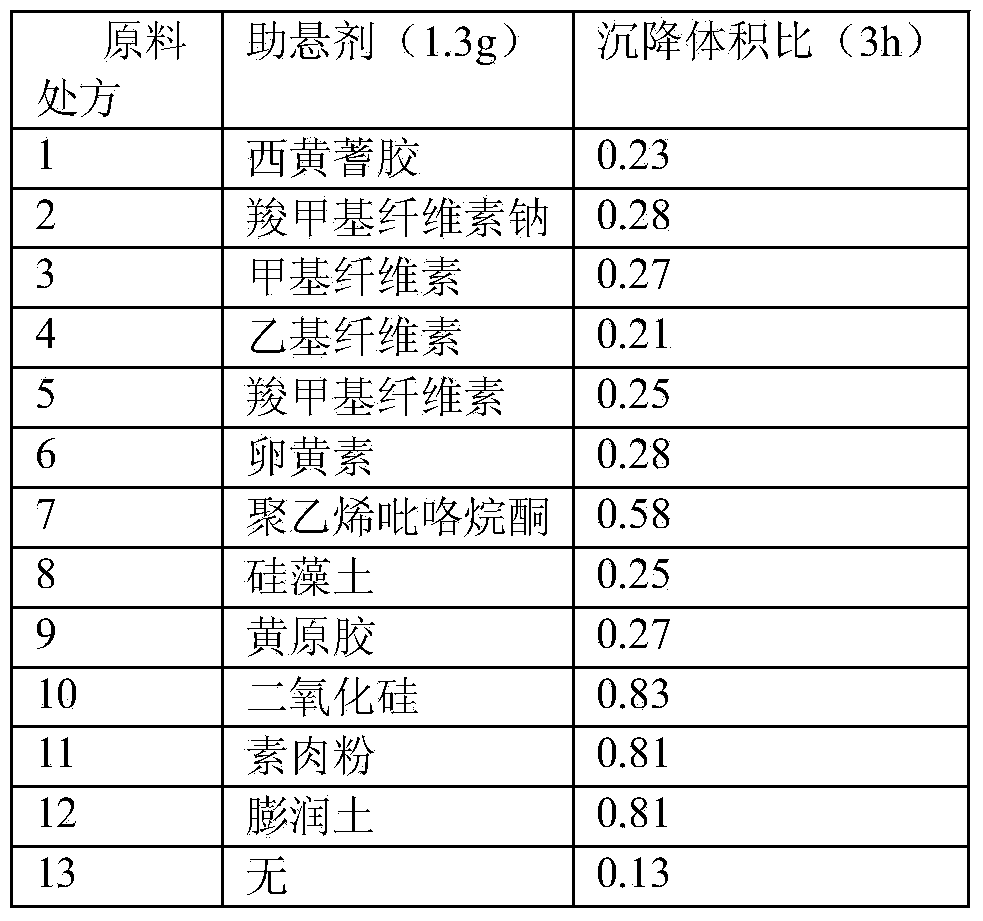
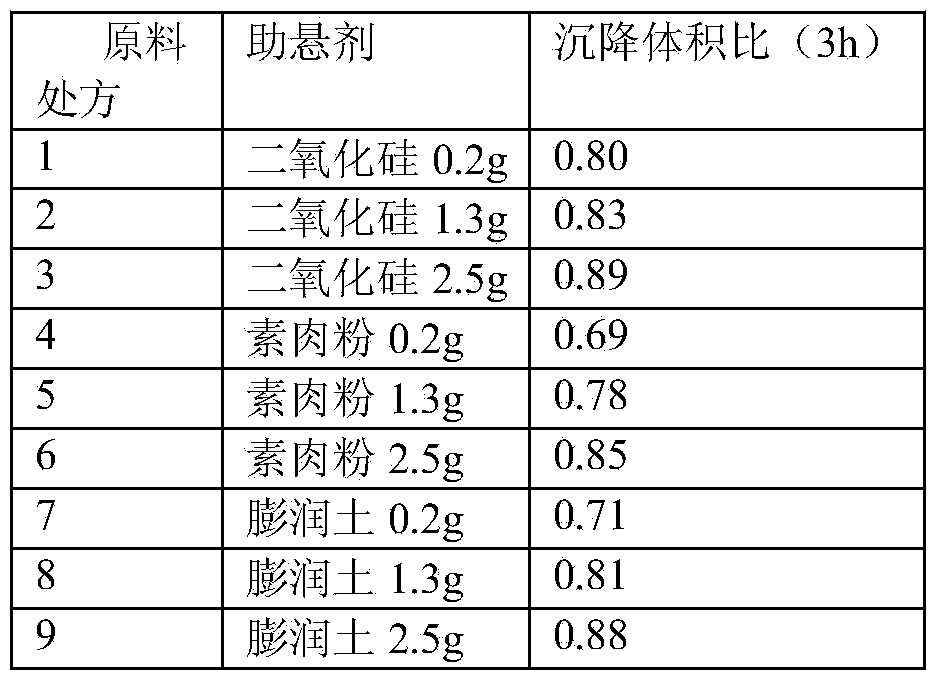
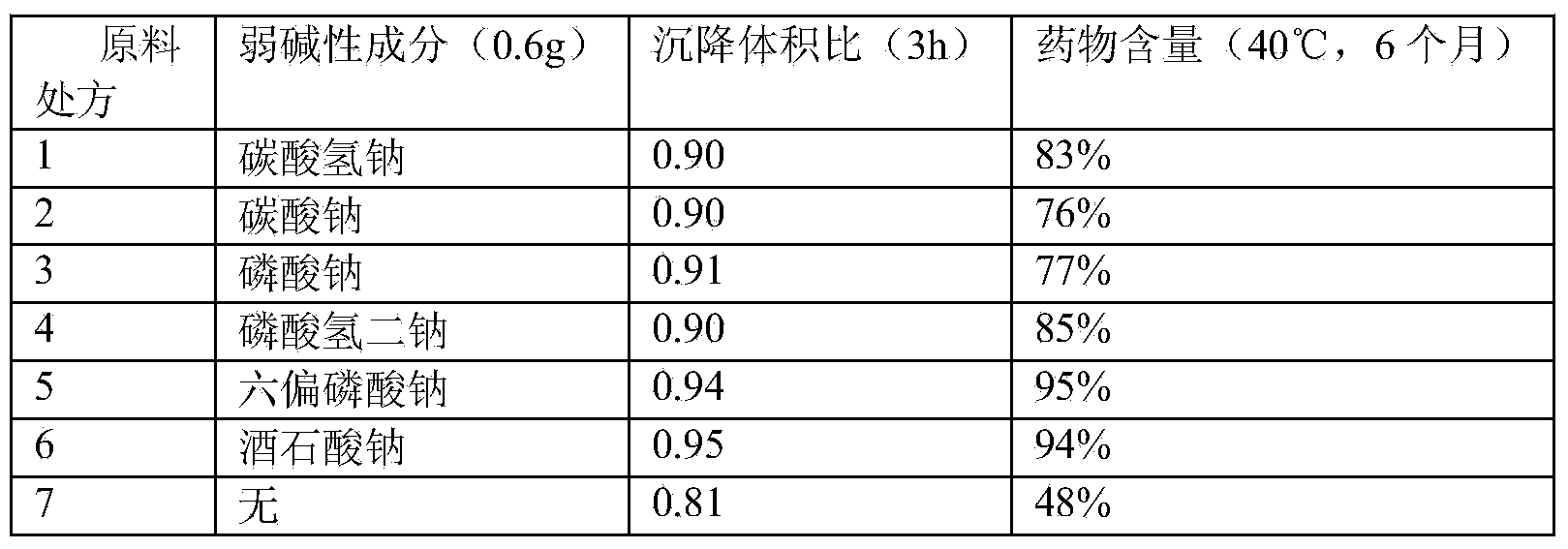
Stable cefquinome sulfate oil suspension injection
A technology of cefquinoxime sulfate and injection, which is applied in the field of antibacterial drug oil suspension type injection, and achieves the effects of less irritation, simple preparation process and improved dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation:
[0055] (1) Weigh silicon dioxide, Span-80, sodium tartrate, propyl gallate, chlorobutanol and ethyl oleate into a mixing tank according to the above prescription, stir into a uniform suspension, and set aside;
[0056] (2) add prescription quantity cefquinome sulfate in the suspension in (1), put and disperse in the colloid mill;
[0057] (3) Transfer the suspension in (2) to a high-pressure homogenizer for homogenization, sterilize after the particle size is qualified, and fill to obtain cefquinome sulfate oil suspension injection.
[0058] Quality Evaluation:
[0059] The cefquinoxime sulfate oil suspension type injection of the present invention is placed 3h and the settling volume ratio is 92%, illustrates that the settling velocity of the suspension is relatively slow; The redispersibility test result shows that the suspension is easily dispersed after shaking, and does not agglomerate; 90% of drug particles in the suspension have a particle...
Embodiment 2
[0064] Preparation
[0065] (1) Weigh cefquinome sulfate, vegetarian meat powder, Tween-80, sodium bicarbonate, vitamin E, phenol and soybean oil for injection according to the above prescription, and mix them uniformly through a colloid mill under sterile conditions After the particle size is qualified, it is filtered and filled to obtain cefquinome sulfate oil suspension injection.
[0066] Quality Evaluation:
[0067] The cefquinoxime sulfate oil suspension type injection of the present invention is placed 3h and the settling volume ratio is 90%, illustrates that the settling velocity of the suspension is relatively slow; The redispersibility test result shows that the suspension is easy to disperse and does not agglomerate after shaking; 90% of the drug particles in the suspension have a particle size of less than 5 μm, 97.8% of which are less than 10 μm, and 100% of which are less than 20 μm; meeting the requirements for suspension injections.
[0068] Stability ...
Embodiment 3
[0072] Preparation
[0073] (1) Filter and sterilize the raw and auxiliary materials in the prescription respectively;
[0074] (2) Under sterile conditions, weigh cefquinoxime sulfate, silicon dioxide, Span-60, sodium tartrate, tert-butyl p-methoxyphenol, phenol and isopropyl myristate respectively according to the above prescription, and mix Uniform;
[0075] (3) The above-mentioned mixture is repeatedly processed by a high-pressure homogenizer, filtered after the particle size is qualified, and filled to obtain cefquinoxime sulfate oil suspension injection.
[0076] Quality Evaluation:
[0077] The cefquinoxime sulfate oil suspension type injection of the present invention is placed 3h after the sedimentation volume ratio is 98%, does not settle substantially, illustrates that the suspension liquid sedimentation velocity is slower; The heavy dispersibility test result shows that the suspension liquid is easy to disperse after shaking, does not Agglomeration; 90% o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com