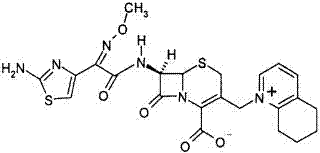
Cefquinome sulfate injection and low-temperature high-shear preparation method thereof
A technology for cefquinoxime sulfate and injection, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. and other problems, to achieve the effect of simple preparation steps and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prepare cefquinome sulfate injection, comprising the steps:
[0036] (1) Weigh each component according to the following mass percentage, and set aside;
[0037] Cefquinome Sulfate 2.3%,
[0038] Lecithin 1.0%,
[0039] Aluminum stearate 2.0%,
[0040] The balance is ethyl oleate;
[0041] (2) High temperature sterilization and cooling of auxiliary materials;
[0042] Add 85% ethyl oleate into the liquid mixing tank, then add the formulated amount of aluminum stearate and lecithin, and stir evenly; heat to 121°C, keep constant temperature, and continue stirring for 30 minutes (the maximum temperature should not exceed 130°C) ; Circulate cold water into the interlayer of the liquid distribution tank, after cooling down to 50°C, slowly add the prescribed amount of cefquinoxime sulfate, and stir until uniform while adding; then use ethyl oleate to make up the volume, and stir evenly;
[0043] (3) Cefquinome sulfate is subjected to low temperature and high shear to obt...
Embodiment 2
[0048] Prepare cefquinome sulfate injection, take each component by the following mass percentages, and set aside;
[0049] Cefquinome Sulfate 2.4%,
[0050] Lecithin 1.0%,
[0051] Aluminum stearate 2.0%,
[0052] Add ethyl oleate to the full amount;
[0053] All the other preparation methods are the same as in Example 1.
Embodiment 3
[0055] Prepare cefquinome sulfate injection, take each component by the following mass percentages, and set aside;
[0056] Cefquinome Sulfate 2.5%,
[0057] Lecithin 1.0%,
[0058] Aluminum stearate 2.0%,
[0059]Add ethyl oleate to the full amount;
[0060] All the other preparation methods are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com