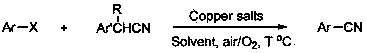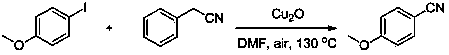Method for preparing aryl nitrile by aryl halogenide
A technology of aryl halides and aryl nitriles, which is applied in the field of preparation of aryl nitriles, can solve the problems of high toxicity of cyanide reagents, complicated separation and purification, and difficult availability of raw materials, and achieve simple steps, good solubility, and easy products Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The method for preparing 4-methoxybenzonitrile by 4-iodoanisole and phenylacetonitrile
[0019]
[0020] The following steps are adopted: add 0.117 grams of p-iodoanisole in the above reaction formula to a 20ml reaction test tube, add 0.088 grams (1.5 equivalents) of phenylacetonitrile, add 0.072 grams (1 equivalents) of cuprous oxide, and add N,N-dimethyl 2 ml of methyl formamide, heated to 130° C., and reacted under air condition for 8 hours. Then cool to room temperature, pour the reaction mother liquor into 15ml of water, extract three times with dichloromethane (3x5ml), combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation. and then separated by column chromatography. 0.061 g of white powdery solid was obtained with a yield of 92%. 1 H NMR (400 MHz, CDCl 3 ): δ 7.59 (d, J = 8.6 Hz, 2H), 6.96 (d, J = 8.6 Hz, 2H), 3.86 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ): δ 163.1, 134.2, 119.5, 115.0, 104.2, 55.8. EIM...
Embodiment 2
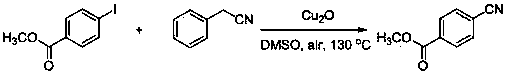
[0022] The method for preparing methyl 4-cyanobenzoate by methyl 4-iodobenzoate and phenylacetonitrile
[0023]
[0024] The following steps are adopted: add 0.131 grams of methyl p-iodobenzoate in the above reaction formula to a 20 ml reaction test tube, add 0.088 grams (1.5 equivalents) of phenylacetonitrile, add 0.072 grams (1 equivalents) of cuprous oxide, and add dimethyl sulfoxide 2 ml, heated to 130°C, and reacted under air conditions for 16 hours. Then cool to room temperature, pour the reaction mother liquor into 15ml of water, extract three times with dichloromethane (3x5ml), combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation. and then separated by column chromatography. 0.069 g of white powdery solid was obtained with a yield of 86%. 1 H NMR (400 MHz, CDCl 3 ): δ 8.15 (d, J = 8.4 Hz, 2H), 7.76 (d, J = 8.4 Hz, 2H), 3.97 (s, 3H). 13 C NMR (100 MHz, CDCl 3 ): δ 165.4, 133.9, 132.2, 130.0, 117.9, 116.3...
Embodiment 3
[0026] Method for preparing 3-cyanoindole from N-methoxyacyl-3-iodoindole and phenylacetonitrile
[0027]
[0028] The following steps are adopted: add 0.151 grams of N-methoxyl-3-iodoindole in the above reaction formula to a 20ml reaction test tube, add 0.088 grams (1.5 equivalents) of phenylacetonitrile, add 0.072 grams (1 equivalents) of cuprous oxide, and add 2 ml of N-methylpyrrolidone was heated to 130° C. and reacted under air conditions for 16 hours. Then cool to room temperature, pour the reaction mother liquor into 15ml of water, extract three times with dichloromethane (3x5ml), combine the organic phases, dry over anhydrous sodium sulfate, and remove the solvent by rotary evaporation. and then separated by column chromatography. 0.046 g of white powdery solid was obtained with a yield of 65%. 1 H NMR (400 MHz, CDCl 3 ): δ 8.80 (br, 1H), 7.80–7.74 (m, 2H), 7.49 (d, J = 7.6 Hz, 1H), 7.37–7.29 (m, 2H). 13 C NMR (125 MHz, d 6 -DMSO): δ 135.9, 135.1, 127.3, 124...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com