Cefquinome sulfate lipidosome and preparation method thereof
A technology of cefquinol sulfate and quinol ester, which is applied in the direction of liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the problems of low solubility, limited use and drug efficacy of cefquinol sulfate. Poor chemical stability and other problems, to achieve the effect of improving lung targeting, obvious lung targeting function, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
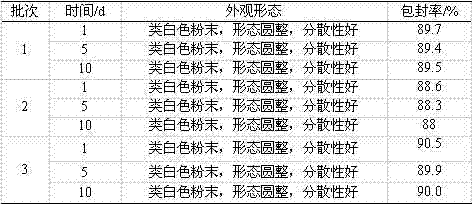
Examples
Embodiment 1 preparation example 1
[0038] Cefquinol Sulfate Liposomal Prescription
[0039] Cefquinol Sulfate 100g
[0041] Cholesterol 150g
[0042] Vitamin E 10g
[0043] Sorbitol 50g
[0044] Cefquinol sulfate liposome preparation method is as follows
[0045] (1) Micronize cefquinol sulfate and pass through a 100-300 mesh sieve;
[0046] (2) Weigh egg yolk lecithin, cholesterol, and vitamin E in the prescribed amount, add 1500 mL of chloroform, stir in a water bath at 70°C, stir to dissolve, and evaporate on a rotary evaporator to make the chloroform solution of egg yolk lecithin, cholesterol, and vitamin E Form a film on the wall, and remove chloroform under reduced pressure;
[0047] (3) Weigh the prescribed amount of micronized cefquinol sulfate, fully dissolve it in 1000 mL of phosphate buffer with a pH value of 7.0, add the prescribed amount of sorbitol, and mix well;
[0048] (4) Add the product obtained in step (3) to the product obtained in step (2), rotate and...
Embodiment 2 preparation example 2
[0050] Cefquinol Sulfate Liposomal Prescription
[0051] Cefquinol Sulfate 100g
[0052] Soy Lecithin 600g
[0053] Cholesterol 200g
[0054] Vitamin E 15g
[0055] Tween 80 100g
[0056] Cefquinol sulfate liposome preparation method is as follows
[0057] (1) Powder cefquinol sulfate and pass through a 100-300 mesh sieve;
[0058] (2) Weigh the prescribed amount of soybean lecithin, cholesterol and vitamin E, add 1500mL of absolute ethanol, stir in a water bath at 80°C to dissolve, and rotate on a rotary evaporator to make soybean lecithin, cholesterol and vitamin E The water-ethanol solution forms a film on the wall, and the absolute ethanol is removed under reduced pressure;
[0059] (3) Weigh the prescribed amount of micronized cefquinol sulfate, fully dissolve it in 1500 mL of phosphate buffer with a pH value of 7.0, add the prescribed amount of Tween 80, and mix well;
[0060] (4) Add the product obtained in step (3) to the product obtained in step (2), rotate and...
Embodiment 3 preparation example 3
[0062] Cefquinol Sulfate Liposomal Prescription
[0063] Cefquinol Sulfate 80g
[0064] Egg Yolk Phosphatidylglycerol 200g
[0065] Cholesterol 100g
[0066] Vitamin E 3g
[0067] Trehalose 30g
[0068] Cefquinol sulfate liposome preparation method is as follows
[0069] (1) Powder cefquinol sulfate and pass through a 100-300 mesh sieve;
[0070] (2) Weigh egg yolk phosphatidylglycerol, cholesterol, and vitamin E, add 1500mL of acetone, stir in a water bath at 65°C to dissolve, and rotate on a rotary evaporator to make egg yolk phosphatidylglycerol, cholesterol, and vitamin E in acetone The solution forms a film on the wall, and the acetone is removed under reduced pressure;
[0071](3) Weigh the prescribed amount of micronized cefquinol sulfate, fully dissolve it in 1000 mL of phosphate buffer with a pH value of 7.4, add the prescribed amount of trehalose, and mix well;
[0072] (4) Add the product obtained in step (3) to the product obtained in step (2), rotate and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com