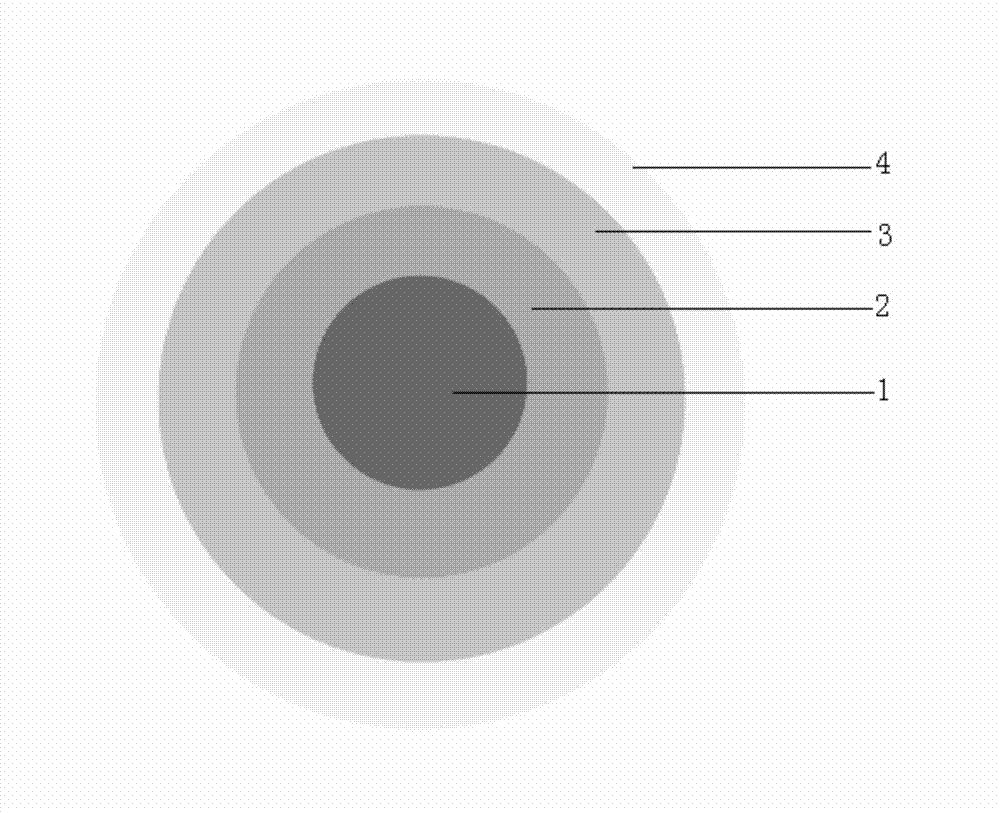
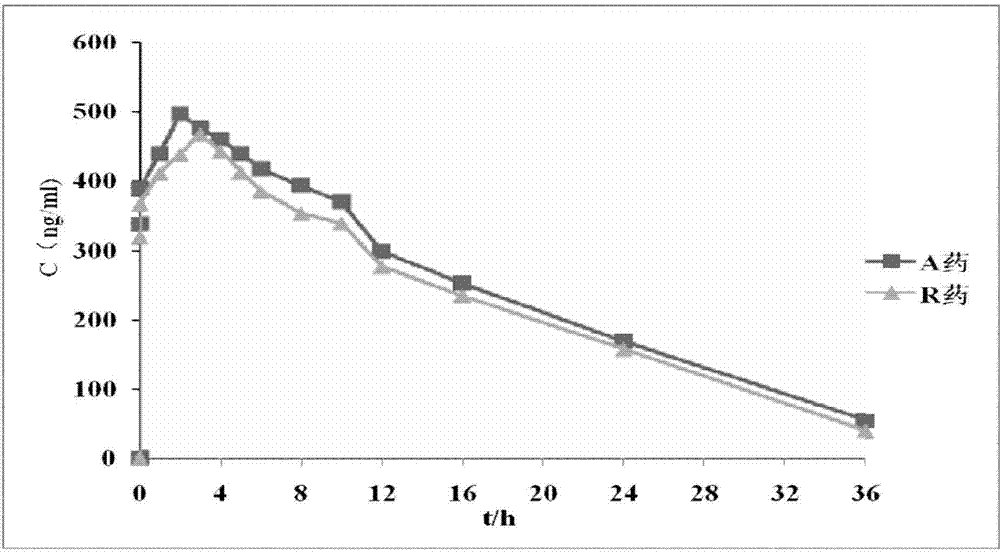
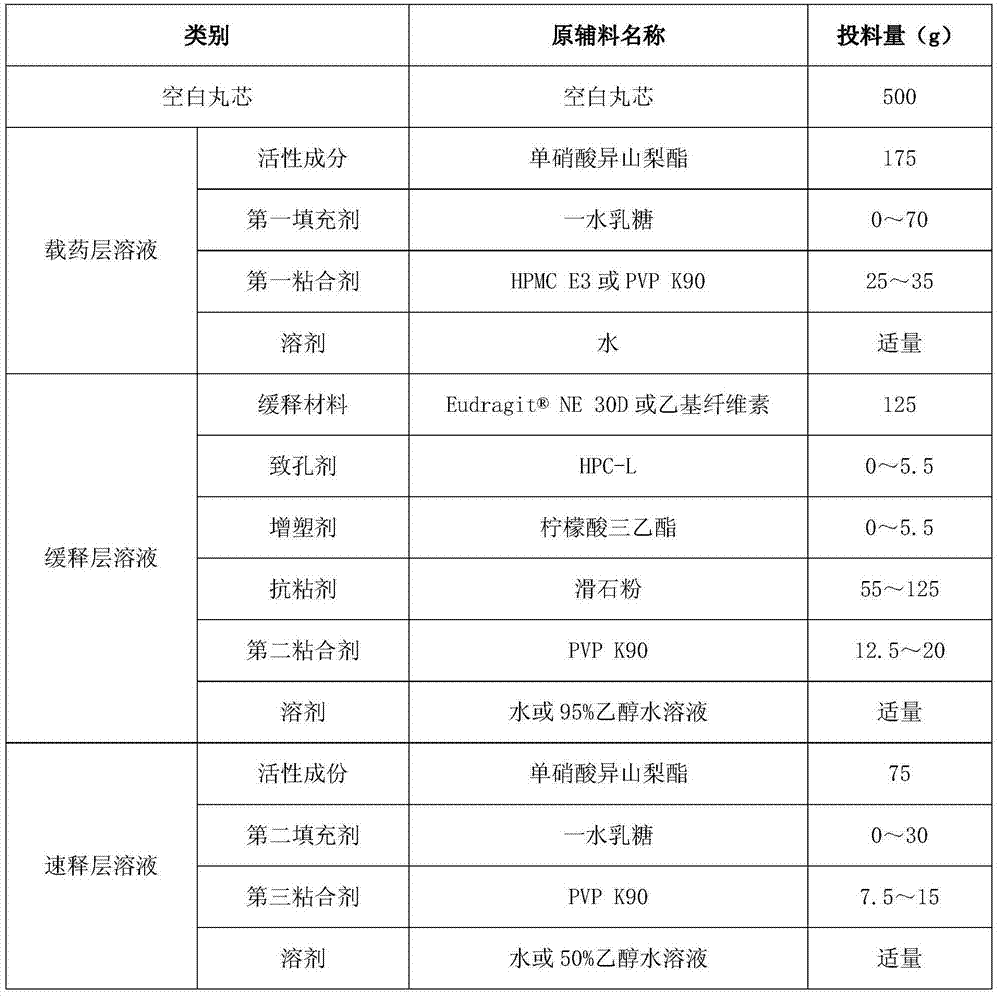
Isosorbide mononitrate sustained-release pallets, preparation prepared from same and preparation method for isosorbide mononitrate sustained-release pallets
A technology of isosorbide mononitrate and sustained-release pellets, which is applied in the field of medicine, can solve the problems of poor stability of isosorbide mononitrate sustained-release capsules, and achieve the effects of avoiding significant changes, simple operation, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] The preparation of embodiment 1 isosorbide mononitrate slow-release pellets
[0111] Add 25g of HPMC E3 into 500g of purified water at 50°C, stir and swell until clear, and cool to the greenhouse for use; add 175g of isosorbide mononitrate, stir evenly, and obtain a suspension. Pass the above suspension through an 80-mesh sieve, rinse it with 250 g of purified water, add 500 g of purified water, and stir evenly to obtain a drug-loaded layer solution. Fill 500g of sucrose-starch microspheres (particle size 0.6~0.8mm) into the fluidized bed granulation coating machine, fan frequency 20~40Hz, air inlet temperature 50~70℃, material temperature 40~50℃, atomization The air pressure is 0.05-0.10MPa, and the spraying pump speed is 10-30 rpm to carry out the drug feeding operation (coating) until the solution of the drug-loaded layer is sprayed out. Set the material temperature at 40°C, fluidize and dry the pellets coated with the drug-loaded layer solution for 2 hours until th...
Embodiment 2
[0118] The preparation of embodiment 2 isosorbide mononitrate slow-release pellets
[0119] Add 25g of HPMC E3 to 500g of purified water at 50°C, stir and swell to clarification, and cool to the greenhouse for use; take 43.8g of lactose monohydrate, add to the above solution and stir to dissolve; add 175g of isosorbide mononitrate, stir evenly, and obtain suspension. Pass the above suspension through an 80-mesh sieve, rinse it with 250 g of purified water, add 500 g of purified water, and stir evenly to obtain a drug-loaded layer solution. Fill 500g of sucrose-starch microspheres (particle size 0.6~0.8mm) into the fluidized bed granulation coating machine, fan frequency 20~40Hz, air inlet temperature 50~70℃, material temperature 40~50℃, atomization The air pressure is 0.05-0.10MPa, and the spraying pump speed is 10-30 rpm to carry out the drug feeding operation (coating) until the solution of the drug-loaded layer is sprayed out. Set the material temperature at 40°C, fluidiz...
Embodiment 3
[0126] The preparation of embodiment 3 isosorbide mononitrate slow-release pellets
[0127] Add 25g of PVP K90 to 500g of purified water at 25°C, stir and dissolve until clear and ready for use; take 43.8g of lactose monohydrate, add to the above solution and stir to dissolve; add 175g of isosorbide mononitrate, stir well to obtain a suspension. Pass the above suspension through an 80-mesh sieve, rinse it with 250 g of purified water, add 500 g of purified water, and stir evenly to obtain a drug-loaded layer solution. Fill 500g of sucrose-starch microspheres (particle size 0.6~0.8mm) into the fluidized bed granulation coating machine, fan frequency 20~40Hz, air inlet temperature 50~70℃, material temperature 40~50℃, atomization The air pressure is 0.05-0.10MPa, and the spraying pump speed is 10-30 rpm to carry out the drug feeding operation (coating) until the solution of the drug-loaded layer is sprayed out. Set the material temperature at 40°C, fluidize and dry the pellets c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com