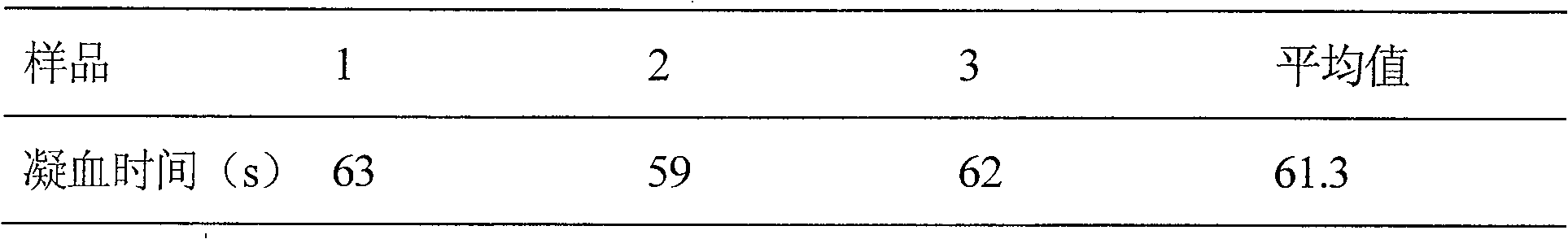Compound stypticum
A drug and compound technology, applied in the field of medicine, can solve problems such as adverse immune response, limited dosage, glomerular damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
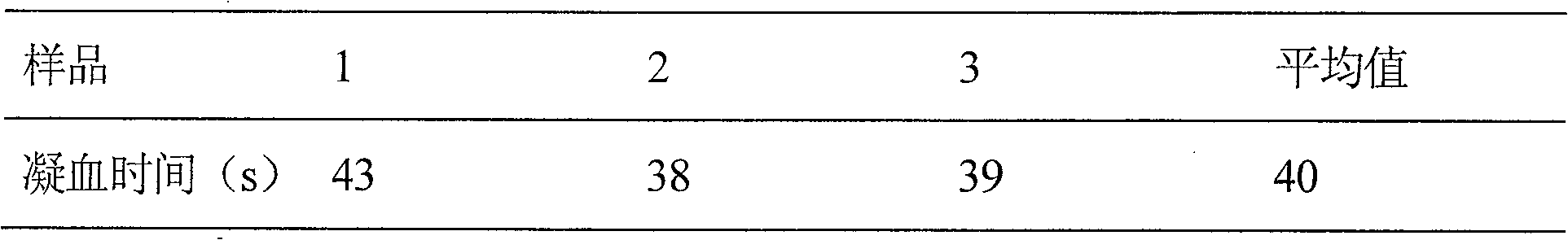
Embodiment 1
[0020] Prescription: thrombin-like enzyme 50u
[0021] IL-11 500mg
[0022] Glycine 1000mg
[0023] Mannitol 4000mg
[0024]
[0025] Add water for injection to 200ml
[0026] The thrombin in the prescription is the thrombin-like batroxobin contained in the venom of Agkistrodon lancehead, and rhIL-11 is recombinant human interleukin-11.
[0027] Preparation process: Weigh the prescription amount of raw materials and auxiliary materials in a clean room, put them in a sterilized container, add 200ml of sterilized water for injection, stir to dissolve, add 1% of the prescription amount of activated carbon for needles, stir well, and let stand for 30min , filtered through a 0.8 μm microporous membrane filter, and then filtered through a 0.22 μm microporous membrane sterilizer, and then packed in sterilized vials. Pre-freeze at -30°C-40°C for 2-3 hours, sublimate and dry at -20°C for 9 hours, dry at 30°C for ...
Embodiment 2
[0031] Prescription: thrombin-like enzyme 50u
[0032] IL-11 500mg
[0033] Glycine 1000mg
[0034] Mannitol 4000mg
[0035]
[0036] Add water for injection to 200ml
[0037] The thrombin-like enzyme in the prescription is the thrombin-like enzyme contained in the venom of Agkistrodon halys, and the IL-11 is recombinant human interleukin-11.
[0038] Preparation process: Weigh the prescription amount of raw materials and auxiliary materials in a clean room, put them in a sterilized container, add 200ml of sterilized water for injection, stir to dissolve, add 1% of the prescription amount of activated carbon for needles, stir well, and let stand for 30min , filtered through a 0.8 μm microporous membrane filter, and then filtered through a 0.22 μm microporous membrane sterilizer, and then packed in sterilized vials. Pre-freeze at -30°C-40°C for 2-3 hours, sublimate and dry at -20°C for 9 hours, dry at 30°C fo...
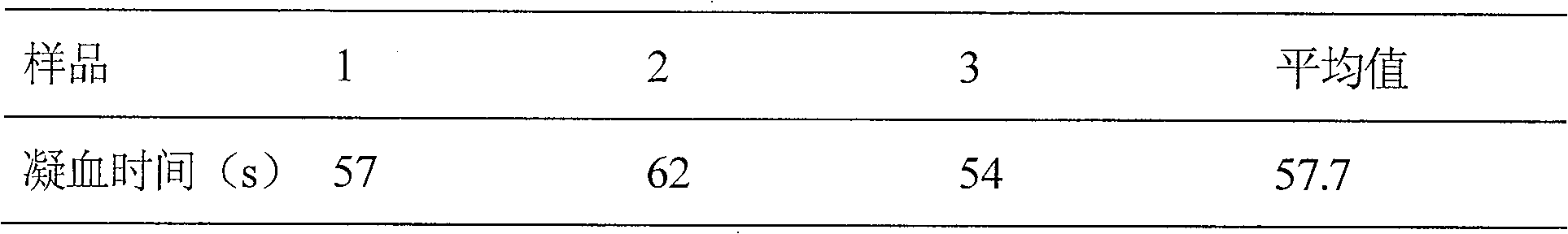
Embodiment 3
[0042] Prescription: thrombin-like enzyme 50u
[0043] IL-11 500mg
[0044] Glycine 1000mg
[0045] Mannitol 4000mg
[0046]
[0047] Add water for injection to 200ml
[0048] The thrombin-like enzyme in the prescription is the thrombin-like enzyme contained in the venom of Agkistrodon akistrodon, and the IL-11 is recombinant human interleukin-11.
[0049] Preparation process: Weigh the prescription amount of raw materials and auxiliary materials in a clean room, put them in a sterilized container, add 200ml of sterilized water for injection, stir to dissolve, add 1% of the prescription amount of activated carbon for needles, stir well, and let stand for 30min , filtered through a 0.8 μm microporous membrane filter, and then filtered through a 0.22 μm microporous membrane sterilizer, and then packed in sterilized vials. Pre-freeze at -30°C-40°C for 2-3 hours, sublimate and dry at -20°C for 9 hours, dry a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com