Stable Batroxobin medicament composition
A composition and technology of batroxobin, applied in the field of medicine, can solve the problems of increasing the potential danger of exogenous viruses, increasing production costs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1—7
[0088] Preparation of batroxobin liquid preparation
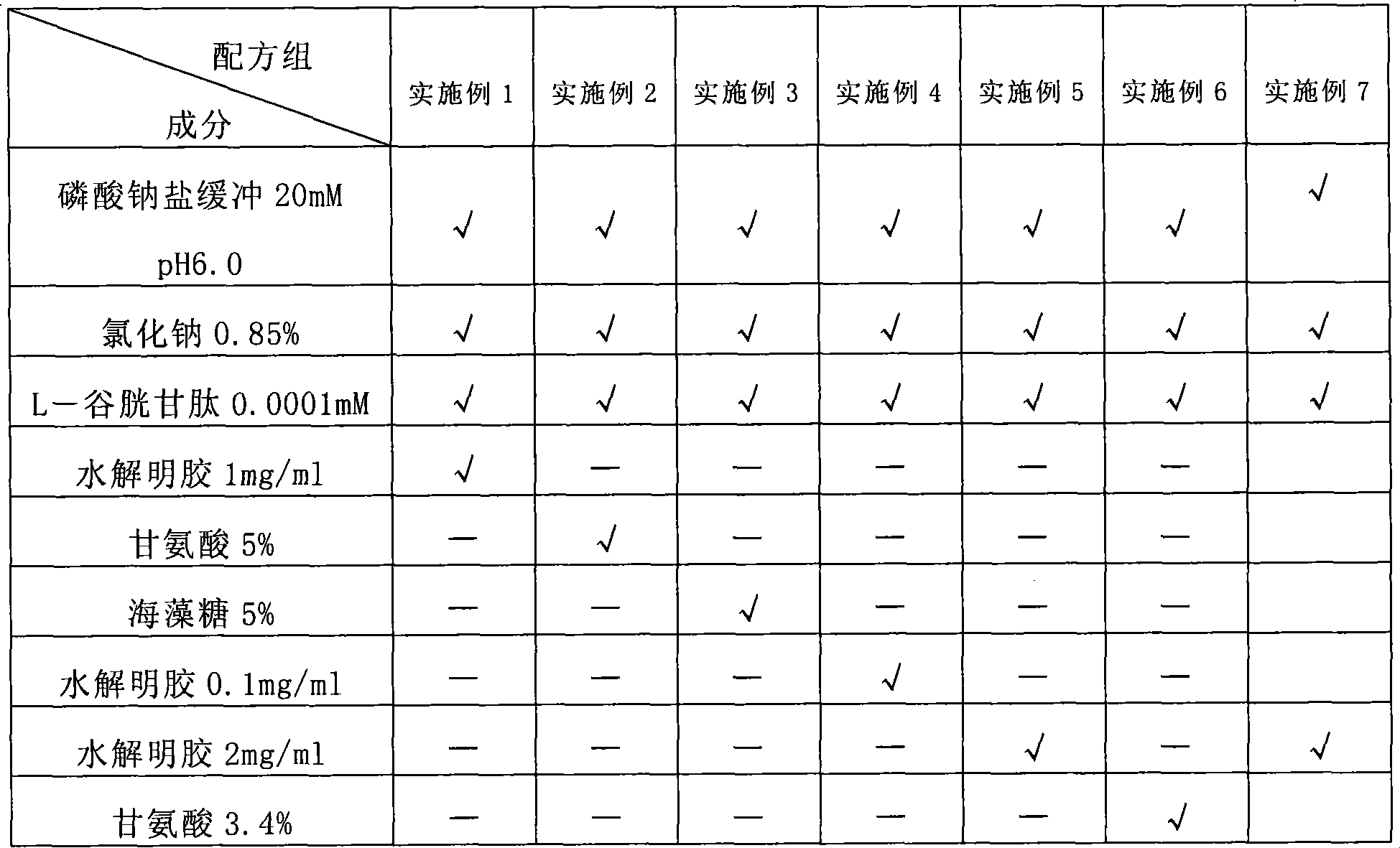
[0089] The constructed batroxobin enzyme methanol yeast engineering strain (CCTCC No: M203006) was fermented in a tank, and after a series of chromatographic purification steps, a highly purified gene recombinant batroxobin enzyme and injection containing the various excipient components in the following table were obtained. The aqueous solution is mixed to obtain the recombinant batroxobin liquid preparation.
[0090]
[0091]
[0092] √ means that this auxiliary material is selected for this group of formulas.
Embodiment 9—15
[0109] Example of Stability Test
[0110] According to the screening results of the above buffer system, the phosphate buffer with pH 6.0 was selected as the basic buffer system, and various stabilizing and protecting agents were added for stability investigation.
[0111] Referring to the composition of the formula of embodiment 1-7:
[0112]
[0113] √ means that this auxiliary material is selected for this group of formulas.
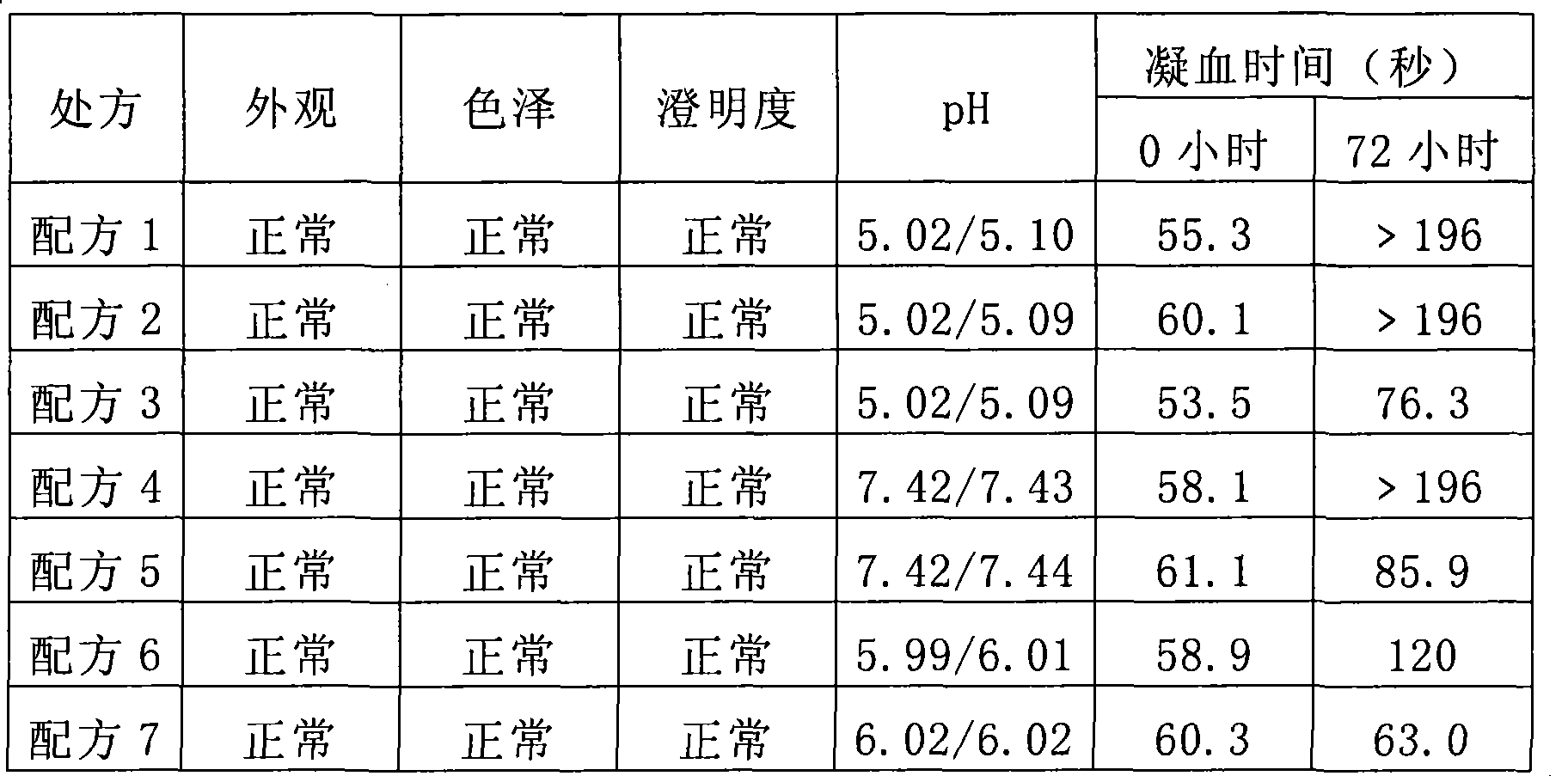
[0114] 37°C stability test results
[0115] The specifications of the recombinant batroxobin preparations of Examples 1-7 are all 1KU / ml, that is to say, at each check point, 100ul of a solution containing a certain formula of recombinant batroxobin is mixed with 100ul of anticoagulated human standard plasma Mixed, at 37°C, it can solidify within 60±20 seconds. Anything out of range is considered unqualified.
[0116] When carrying out the stability test of the preparation, the stock solution is diluted to an active concentration of 1KU / ml.
[0...
Embodiment 16
[0123] Example of Stability Test
[0124] Using the formula in Example 7, a high-concentration recombinant batroxobin stock solution (recombinant batroxobin protein concentration of 2.5 mg / ml) was preserved.
[0125] The recombinant batroxobin solution obtained through chromatographic purification was passed through a G25 desalting chromatographic column to replace the buffer system of the recombinant batroxobin to test the effect of the formula on protecting the recombinant batroxobin stock solution or high-concentration recombinant batroxobin. The specific operation is as follows:
[0126] Step 1: Equilibrate about 4 column bed volumes of the G25 chromatographic column with the solution prepared by supplementing the components and contents as in Example 7;
[0127] Step 2: Put the recombined batroxobin solution in the amount of 1 / 4 column bed volume, carry out desalting chromatography treatment, collect the absorption peak part of the effluent, at this time the recombined b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com