Site-directed mutagenesis genetic engineering batroxobin and uses thereof
A site-directed mutagenesis and genetic engineering technology, applied in the field of batroxobin, can solve problems such as proteins with complex sugar chains in spatial structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] According to the nucleic acid sequence of batroxobin (X12747) published in the U.S. Genebank, select the genetic codes that yeast likes, artificially synthesize the structural gene sequence of the site-specific transformation of batroxobin, and mutate the code AGA of the 45th Arg into AAG encoding Lys, the yeast endogenous KEX2 protease recognition sequence Arg-Arg is mutated to Arg-Lys, so KEX2 protease will not degrade batroxobin secreted into the fermentation broth at this site, so that the target protein can be produced in large quantities accumulation. In order to recombine the synthetic target gene into the yeast secretory expression vector pPICZα, an Xho I restriction site was added to the 5' end of the gene, and a stop codon TAATGA and a SacII restriction site were added to the 3' end of the gene. In addition, the codon AAAAGA corresponding to the KEX2 protease recognition sequence Lys-Arg is added between the Xho I restriction site and the first amino acid Val ...
Embodiment 2
[0067] Linearize pPICZa-Bg with DNA restriction endonuclease SacI, prepare yeast host competent and transform according to the method in Multi-Copy Pichia Expression Kit Version B of Invitrogen Company, and spread the transformed cells on YPD+500ug / ml It grows on Zeocin medium, and a single colony can appear after 3-4 days at 30°C. Clones with large and full colonies were selected for expression screening.
Embodiment 3
[0069] The highly expressed engineered bacteria screened out in test tubes were inoculated into shake flasks for proliferation as seed liquid, and then transferred to a 10L fermenter for pilot-scale fermentation according to the conventional method of methanol yeast fermentation. Induction for 72 hours. Centrifuge the fermentation broth to collect the supernatant, or concentrate and desalt it by ultrafiltration, and use hydrophobic column chromatography, ion exchange column chromatography, affinity column chromatography, gel column chromatography and other biochemical separation techniques to obtain the activity with a purity of more than 97%. protein.
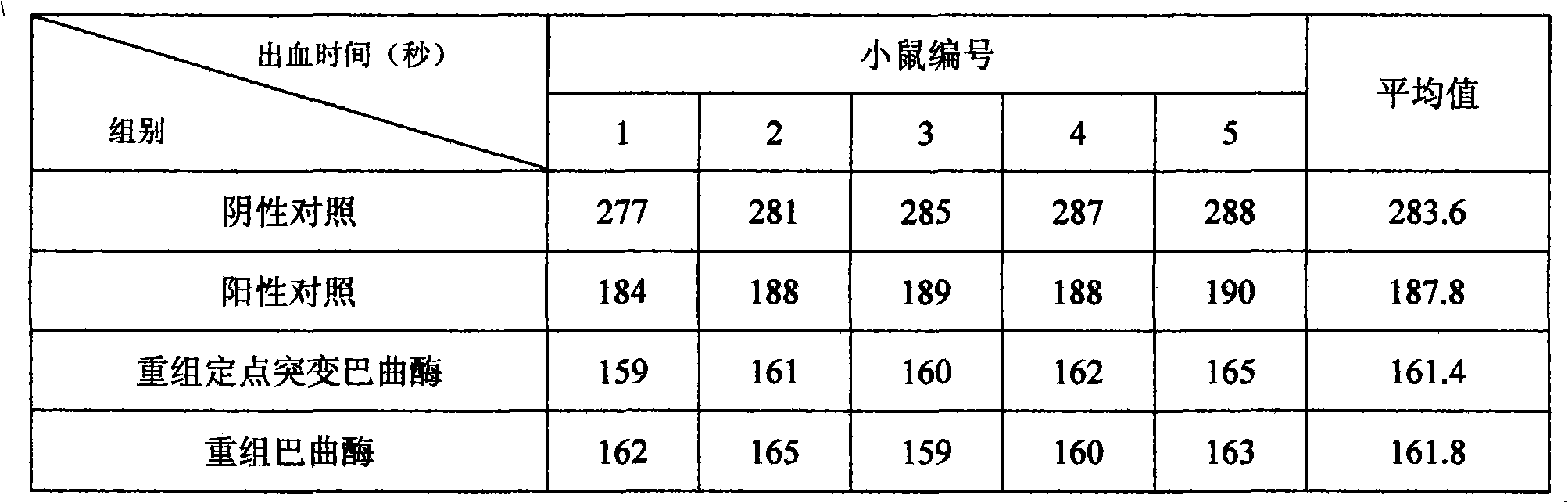
[0070] The method for measuring the activity of similar products was used for the in vitro coagulation test. The specific test method was as follows: take 0.2ml of citrate anticoagulated plasma, put it into a test tube with a diameter of 1cm, put it in a water bath at 37°C for 3 minutes, and add it to preheat at 37°C Mix 0.2m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com