Method for detecting activity of batroxobin
A detection method and technology of batroxobin are applied in the direction of material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., which can solve the problems of large subjective interference, high cost, and restriction of wide application. , to achieve the effect of reducing reading time, easy operation and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] ① Use 0.02M Tris-HCl buffer solution, the pH of the solution is 7.5, prepare 3mM S-2238 substrate solution and 16Bu / ml defibrase standard stock solution;
[0028] ②Randomly select the snake venom hemagglutinase preparation with batch number 20100409 produced by Zhaoke Pharmaceutical (Hefei) Co., Ltd. as a test sample, and the labeling amount is 1Ku / ml;
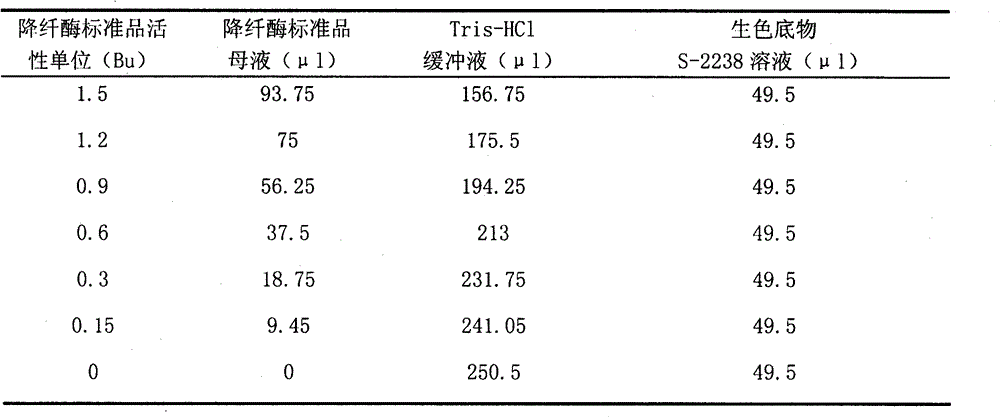
[0029] ③Add defibrase standard mother solution with activity units of 1.5, 1.2, 0.9, 0.6, 0.3, and 0.15Bu to each well of the microtiter plate, make up to 250.5 μl with 0.02M Tris-HCl buffer solution, pH=7.5, Then add 49.5 μl 3mM chromogenic substrate S-2238 solution, mix well, make 3 duplicate wells for each active unit standard product mother solution, and the reaction system is shown in Table 1;
[0030] Table 1 Defibrase standard reaction system
[0031]
[0032] ④ Add 0.2Ku snake venom hemagglutinin preparation to the wells of the microplate, volume 200μl, 49.5μl 3mM chromogenic substrate S-2238 and 50.5μl 0.02...
Embodiment 2
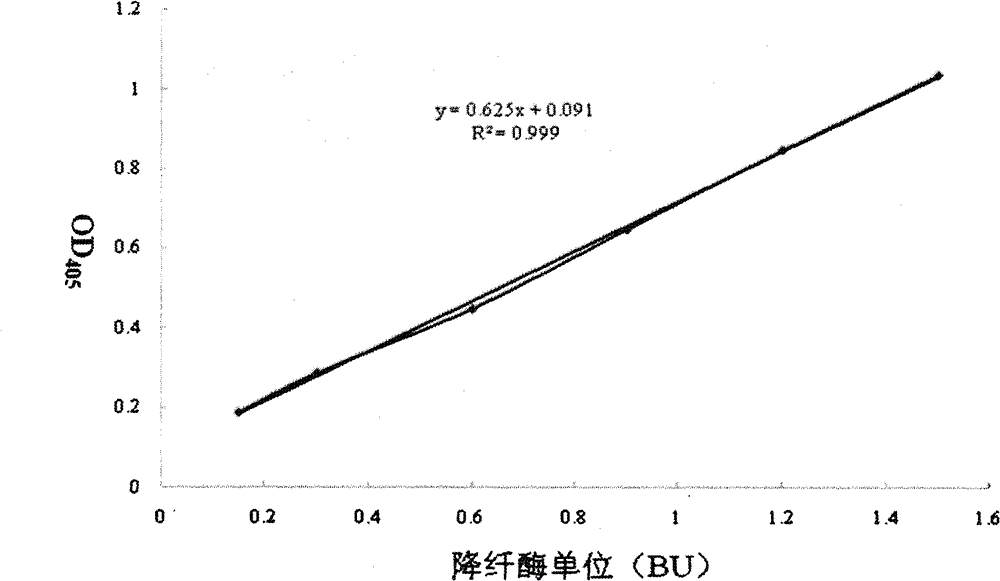
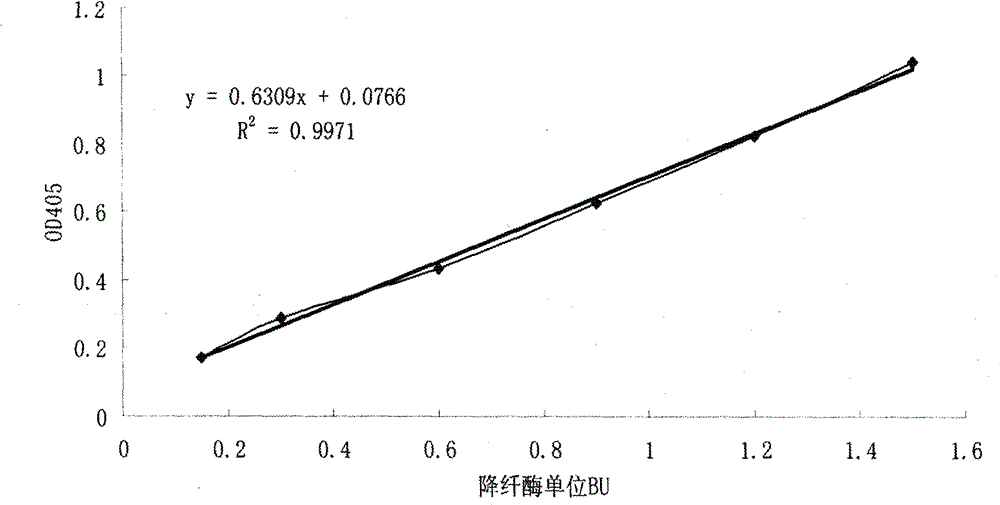
[0035] Select four batches of snake venom hemagglutinase preparations produced by Zhaoke Pharmaceutical (Hefei) Co., Ltd., the batch numbers are 20100315, 20100206 and 20091217, and the specifications are all 1Ku / ml. In the microtiter plate, the standard curve reaction system is the same as in Example 1, with 3 duplicate wells each. The reaction systems of the three batches of samples to be tested were 200 μl of preparation (0.2 Ku / well of enzyme), 50.5 μl of Tris-HCl solution and 49.5 μl of S-2238 substrate, and each sample had 5 duplicate wells. Put the prepared microplate plate into the microplate reader preheated for 4 minutes to 37 ° C for 15 minutes, and measure the absorbance A405 of the enzymatic hydrolysis product p-nitroaniline (PNA) in each hole at a wavelength of 405 nm in parallel to make For the standard curve of enzyme activity, the absorbance A405 value of the sample to be tested was measured, and the activity unit of the sample to be tested was calculated by s...
Embodiment 3
[0039]Take a certain batch of Batroxobin monomer with a protein content of 3.3mg / ml and 3.73mg / ml in the reserved sample after separation and purification from Zhaoke Pharmaceutical (Hefei) Co., Ltd., and use 0.02M Tris-HCl buffer solution, pH=7.5 , which was diluted to 3.3 μg / ml and 3.73 μg / ml. In the microplate plate, the standard curve reaction system was the same as that in Example 1, with 3 duplicate holes each. The reaction systems of the samples to be tested were 24 μl and 21 μl of the preparation mother solution, the amount of enzyme was 0.08 μg / well, 226.5 μl and 229.5 μl of Tris-HCl solution and 49.5 μl of S-2238 substrate, and each sample had 5 replicate wells. Immediately put the above-prepared microplate plate into a microplate reader preheated for 4 minutes to 37 ° C for 15 minutes, and then measure the absorbance A405 of the enzymatic hydrolysis product p-nitroaniline (PNA) in each hole at a wavelength of 405 nm in parallel. Make a standard curve of enzyme activ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com