Method for detecting enzymatic activity of phospholipid-depending factor X activator
A blood coagulation factor and detection method technology, which is applied in the directions of biochemical equipment and methods, microbiological determination/inspection, color/spectral characteristic measurement, etc., can solve problems such as inability to accurately reflect the characteristics of enzyme activity, and achieve easy control and process Simple, efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] ①Use 0.02M Tris-HCl buffer (pH 7.4) to prepare the FXA chromogenic substrate S-2765 into a solution with a concentration of 3mM; accurately measure 0.8ml of the prepared 3mM S-2765 solution and add 0.6ml of 0.075M CaCl 2 solution, mix well and set aside;
[0039] ②Take one piece of Human Factor X (100μg), dilute it with 0.02M Tris-HCl buffer (pH 7.4) to a solution with a concentration of 2.5μM, mix well and set aside;
[0040] ③ Add 10 mg of human serum albumin to 10 ml of 0.02M Tris-HCl buffer (pH 7.4) to prepare a serum albumin dilution with a concentration of 1 mg / ml;
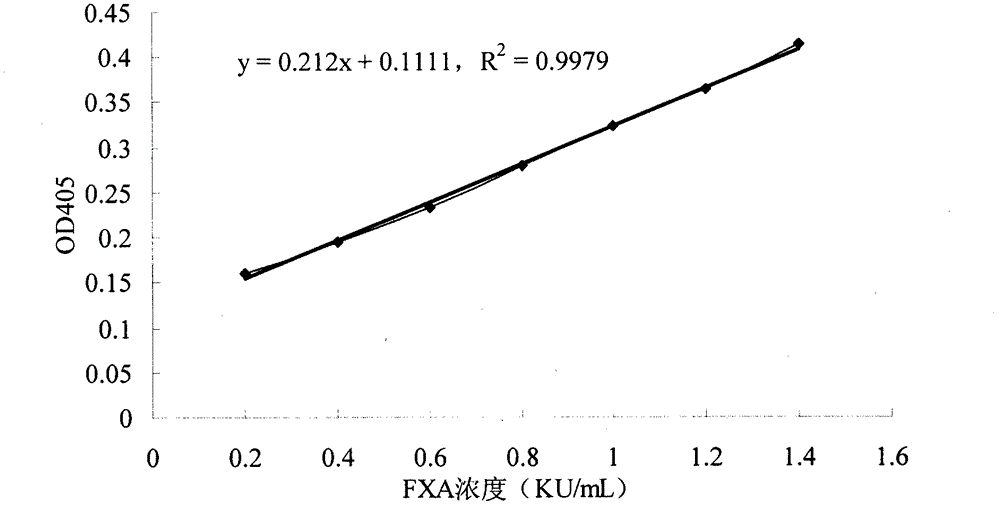
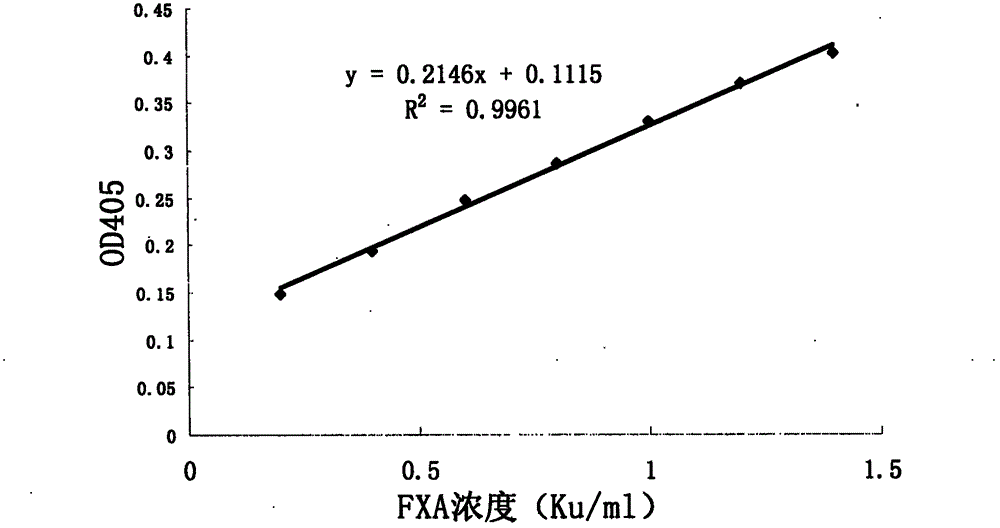
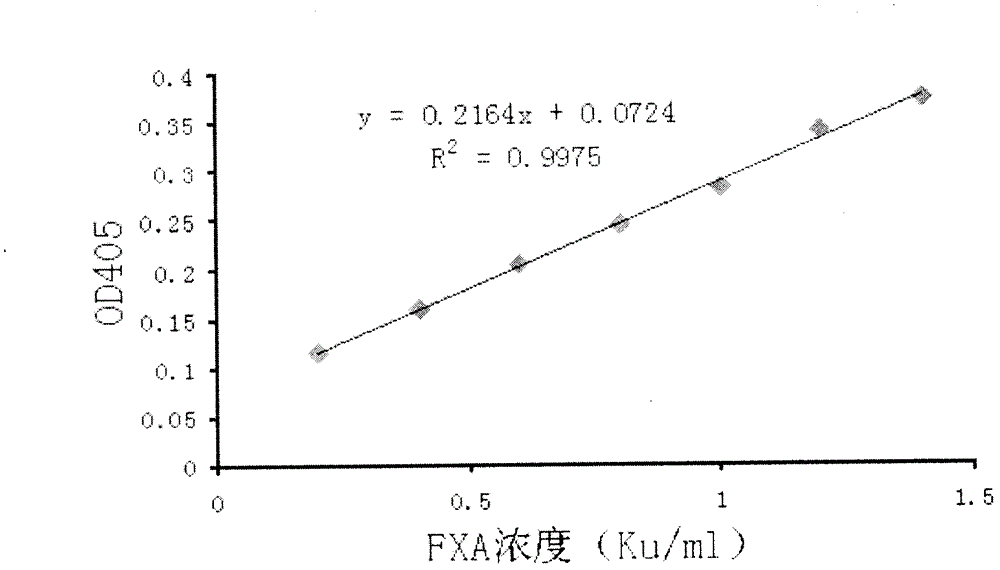
[0041] ④Use the prepared 1mg / ml serum albumin diluent and 0.02M Tris-HCl buffer solution (pH 7.4) to dilute the FXA monomer standard substance into 7 different concentrations of standard substance mother solutions, the concentrations of which are: 1.4, 1.2, 1.0, 0.8, 0.6, 0.4, 0.2 Ku / ml;
[0042] ⑤ Randomly select the snake venom hemocoagulase injection preparation produced by Zhaoke Pharmaceutical ...
Embodiment 2
[0047] Take 10 samples of FXA to be tested with concentrations of 0.34, 0.355, 1.904, 0.421, 0.602, 0.206, 0.477, 0.662, 0.683 and 1.767mg / ml prepared by Zhaoke Pharmaceutical (Hefei) Co., Ltd., respectively, with 0.02M Tris Dilute the batch of test samples with HCl buffer solution and 1 mg / ml serum albumin diluent (the diluted concentration of the batch of test samples is 35 ug / ml). In the microtiter plate, the standard curve reaction system is the same as in Example 1, with 3 duplicate wells each. The reaction system of the sample to be tested was 23 μl of the chromogenic substrate S-2765 solution, 30 μl of the Human Factor X solution, and 7 μl of the sample solution to be tested in each well, and mixed immediately. Put the prepared microplate plate into the microplate reader preheated for 4 minutes to 37 ° C for 15 minutes, and measure the absorbance A405 of the enzymatic hydrolysis product p-nitroaniline (PNA) in each hole at a wavelength of 405 nm in parallel to make For...
Embodiment 3
[0051] Take 20 batches of samples of snake venom hemagglutinin intermediates produced by Zhaoke Pharmaceutical (Hefei) Co., Ltd., the batch numbers are 20080403, 20080404, 20080405, 20080406, 20080407, 20080501, 20080602, 20080605, 20090104, 20090105, 2040203 , 20090402, 20090501, 20090601, 20090702, 20090703, 20091001, 20100401 and 20100501, the specifications are 200, 100 and 400Ku / ml respectively, and the batch is diluted with 0.02M Tris-HCl buffer and 1mg / serum albumin diluent to be tested Sample (the concentration of the batch of samples to be tested after dilution is 0.8Ku / ml). In the microtiter plate, the standard curve reaction system is the same as in Example 1, and each concentration has 3 duplicate wells. The reaction system of the sample to be tested was 23 μl of the chromogenic substrate S-2765 solution, 30 μl of the Human Factor X solution, and 7 μl of the sample solution to be tested in each well, and mixed immediately. Put the prepared microplate plate into th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com