Vector for expressing recombinant blood coagulation factor FIX and construction method and applications thereof
A blood coagulation factor and carrier technology, applied in the field of genetic engineering, can solve the problems of unsafe packaging and complexity of viruses, and achieve the effect of alleviating the bleeding symptoms of hemophilia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, construction and separation and purification of vector
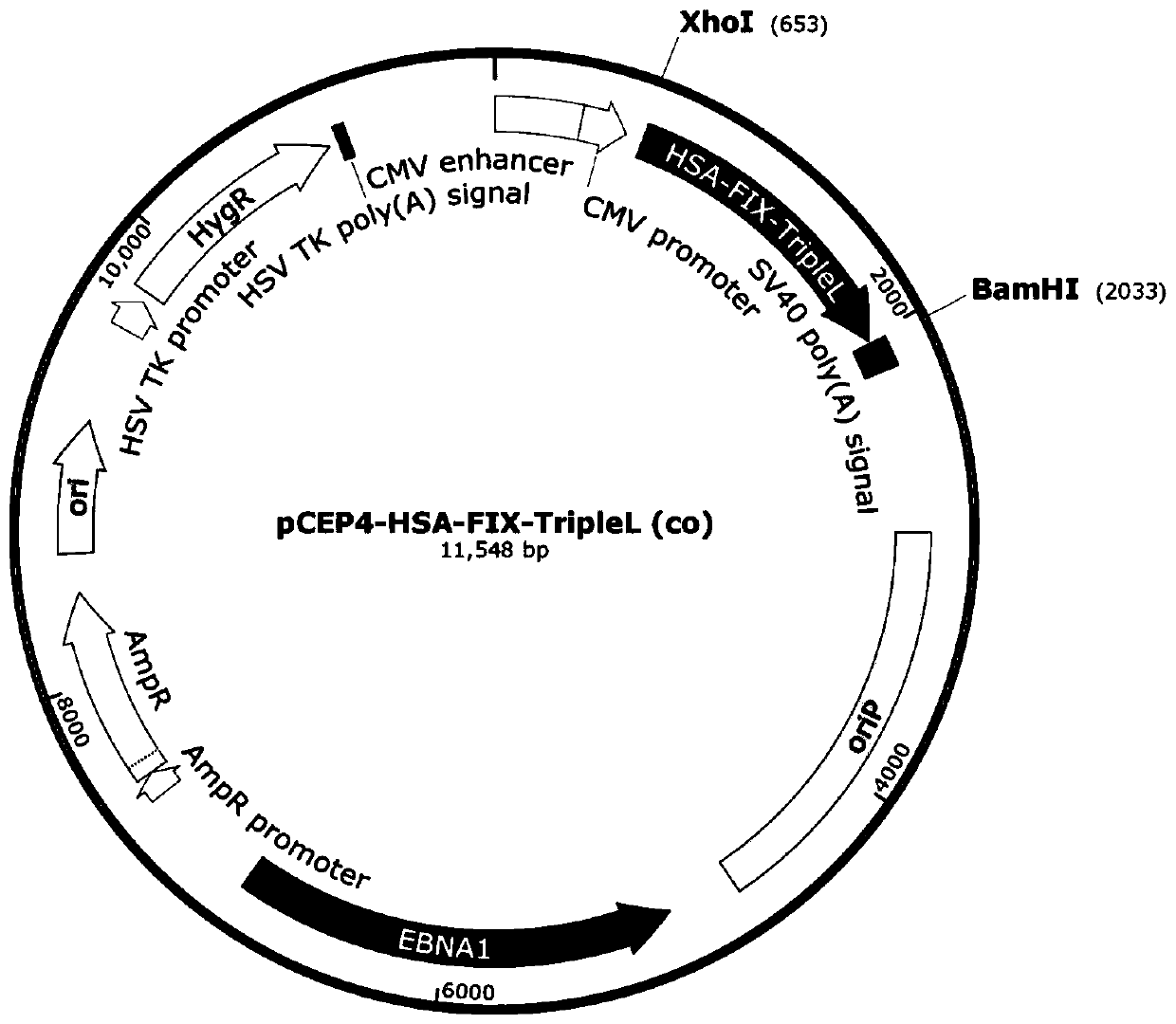
[0027] The plasmid packaged into recombinant FIX is named pCEP4-HSA-FIX-TripleL, and the construction method is as follows figure 1 As shown, the replicon of the expression vector has a human herpes virus replicon (replication origin, OriP), and an EBNA-1 expression cassette, and the cDNA (SEQ ID NO: 1) of the human FIX gene is cloned into the downstream of the CMV promoter , In addition, the plasmid also includes a CMV enhancer and a CMV promoter, both of which can enhance the long-term expression of the transgene FIX. Human coagulation factor genes are synthesized by the company, and the front end of FIX cDNA is the secretion signal peptide coding sequence of HSA (SEQ ID NO: 2).
[0028] The secretion efficiency of recombinant protein varies greatly due to different signal peptides. In order to improve the secretion efficiency of coagulation factors, the present invention replaces the signal pepti...
Embodiment 2
[0036] Embodiment 2, construction required umbilical cord mesenchymal stem cells
[0037] The human umbilical cord mesenchymal stem cells required for the construction of the present invention are prepared and obtained by the following steps:
[0038] Take the 25cm2 culture flask for culturing the 3rd-5th generation human umbilical cord mesenchymal stem cells;
[0039] Wipe around the cap of the culture bottle with an alcohol cotton ball, and then unscrew the cap;
[0040] Aspirate and discard the original culture medium in the culture bottle, wash each bottle with 5ml of PBS buffer twice, and aspirate and discard the washing solution;
[0041] Add 0.5ml of trypsin-EDTA to each bottle and put it in a 37°C incubator for 5 minutes;
[0042] Observe the cells under an inverted microscope, and gently tap the culture bottle with the palm of your hand to ensure that the human umbilical cord mesenchymal stem cells are completely digested;
[0043]Add 2ml of complete medium to each...
Embodiment 3
[0045] Example 3, Vector pCEP4-HSA-FIX-TripleL introduced into cells and expressed
[0046] The cells obtained in Example 2 were resuspended with the electroporation solution, and centrifuged and washed once; the cell suspension was counted and resuspended to a density of 1.0-1.2×10 6 / ml cells; get 0.4ml electroporation cup, add cell suspension 100 μ l to each cup, add the gene carrier pCEP4-HSA-FIX-TripleL plasmid DNA 10 μ g that embodiment 1 obtains; Instrument, select eukaryotic cell mode, voltage 350V, electric shock 60μs, electric shock 1 time. Transfer the electroporated cells into a 25cm2 culture flask, add 5ml of complete medium, and culture at 37°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com