A gene therapy carrier and medicine for retinitis pigmentosa
A vector and viral vector technology, applied in the field of genetic engineering, can solve the problems of low expression efficiency, short duration, cytotoxicity, etc., achieve stable expression, and relieve the effects of retinitis pigmentosa
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1, construction and isolation and purification of viral vector
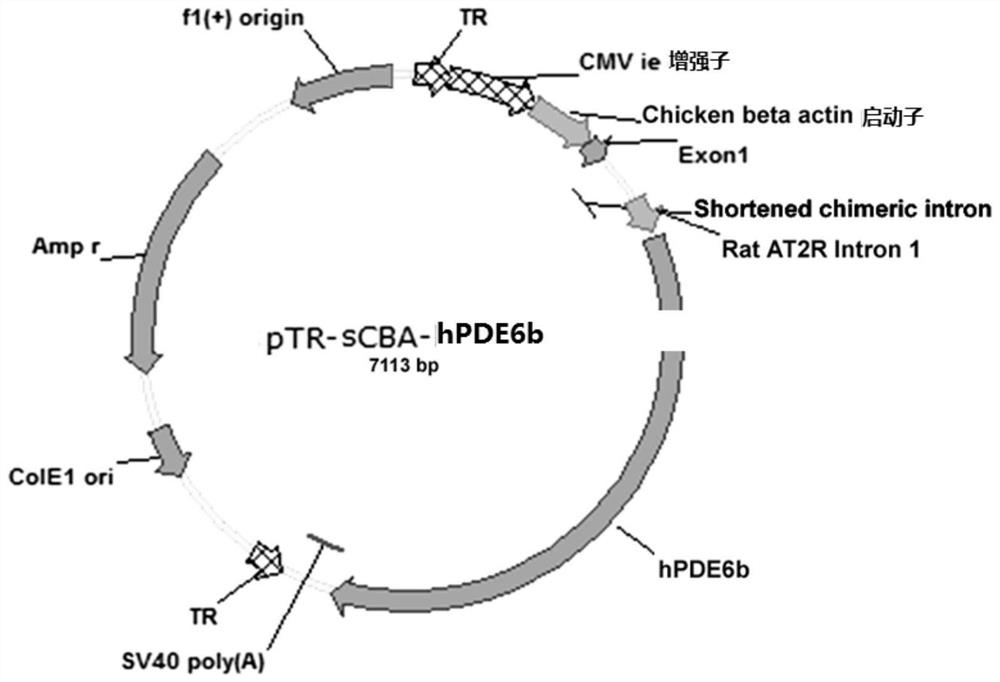
[0058] The plasmid packaged into AAV2 was named pTR-sCBA-AT2R Intron 1-hPDE6b. The construction method of pTR-sCBA-AT2RIntron 1-hPDE6b is as follows figure 1 , figure 2As shown, the cDNA (SEQ ID NO: 1) of human PDE6b (hPDE6b) was cloned into the downstream of the sCBA (shortened chicken beta actin) promoter, and the plasmid also included pTR-sCBA-AT2R Intron 1-hPDE6b IE enhancer ( SEQ ID NO: 5) and the specific rat angiotensin II receptor intron 1 (Rat AT2R Intron 1, SEQ ID NO: 2), both of which can enhance the long-term expression of the transgene PDE6b. The hPDE6b cDNA is followed by the SV40 polyadenylation signal (SEQ ID NO:7) and the expression cassette is flanked by inverted terminal repeats, TR. Viral vectors also include ITR5' (SEQ ID NO:8), ITR3' (SEQ ID NO:9), ColE1ori (SEQ ID NO:10), Amp(r) (SEQ ID NO:11) and f1(+) origin (SEQ ID NO: 12).
[0059] The characteristic map of the v...
Embodiment 2
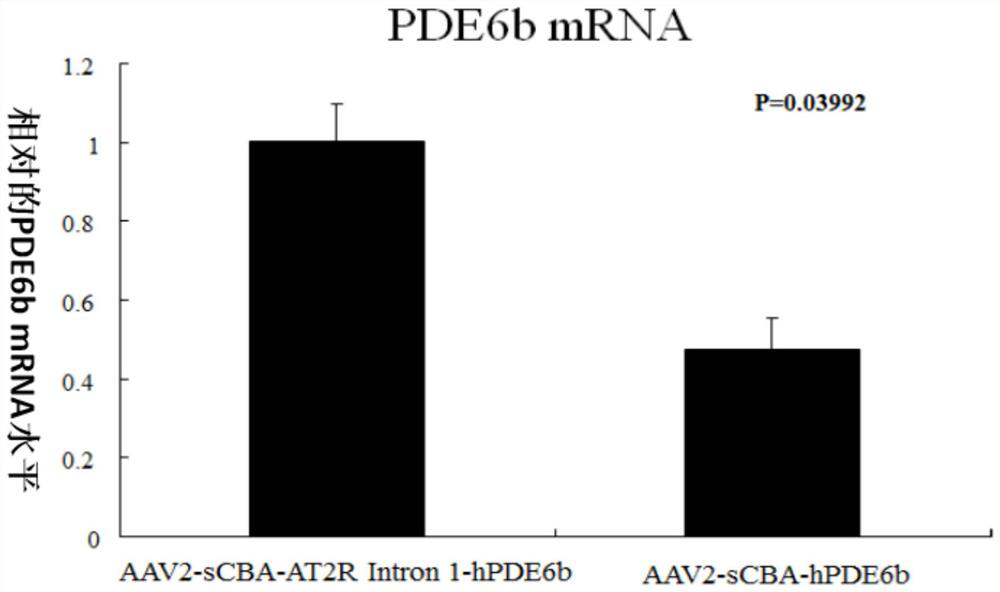
[0061] Example 2, Rat AT2R Intron 1 enhances the expression level of hPDE6b mRNA
[0062] 1. Experimental method
[0063] AAV2-sCBA-hPDE6b with Rat AT2R Intron 1 and AAV2-sCBA-hPDE6b without Rat AT2R Intron 1 were injected into the right and left eyes of the same 14-day-old rd10 mouse. One month later, the eyes were removed for detection mRNA expression levels of hPDE6b.
[0064] 2. Results
[0065] Compared with the traditional vector without Rat AT2R Intron 1, the AAV2-sCBA-hPDE6b viral vector with Rat AT2R Intron 1 (namely AAV2-sCBA-AT2RIntron 1-hPDE6b) can more than double the hPDE6b mRNA level in the injected eyes. ( image 3 )
Embodiment 3
[0066] Embodiment 3, the effect of AAV2-sCBA-AT2R Intron 1-hPDE6b virus vector injection on eye electroretinogram (rod cell ERG)
[0067] 1. Experimental method
[0068] Electroretinogram (ERG) was detected at different time points after subretinal injection of mice. Dark adaptation was carried out overnight. In order to eliminate the influence of detection at different times on the ERG amplitude, the detection was all set in the afternoon and operated under dark red light (>650nm) in a dark room. The mice were placed on a constant temperature platform at 37°C, a drop of compound tropicamide eye drops was instilled on the ocular surface, and then general anesthesia was performed, and the whiskers were cut off. Insert the reference electrode and the ground electrode under the skin of the head and the base of the tail, place a pair of gold ring electrodes on the surface of the cornea, drop a small amount of 2.5% hydroxymethylcellulose eye drops on the surface of the cornea to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com