Compositions comprising factor ó° and factor IXa for treating haemophilia
A composition, hemophilia technology, applied in blood diseases, drug combinations, extracellular fluid diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Materials and methods
[0169] Reagent
[0170] TBS buffer: 50 mM Tris, 150 mM NaCl, 0.02% NaN 2 , pH 7.4, 1% human serum albumin (Zenalb, BPL, Elstree, UK). Bovine fibrinogen (Diagnostic Reagents Ltd. Thame, Oxon UK). Lyophilized reagents from NIBSC, PottersBar, Herts, UK: phospholipids (91 / 542), recombinant FIXa (97 / 562), Agkistrodon antithrombotic enzyme (74 / 581), alpha-thrombin (89 / 588). FIXa reagent was 99% pure and was used to determine the presence or absence of FVIII and tissue factor in a factor Xa production system (Barrowcliffe TW, Fabregas P, Jardi M, Cancelas J, Rabaneda M, and Feize J. Procoagulant activity of Tlymphoblastoid cell due to exposure of negatively charged phosphoripid. Thromb Haemost 2002; 87: 442-449).
[0171] FVIII concentrate
[0172] A series of concentrates obtained with different purities and methods of preparation were studied. Effects The FVIII concentrate (Table 1) was lyophilized material, also obtained from NIBSC as a referen...
Embodiment 2
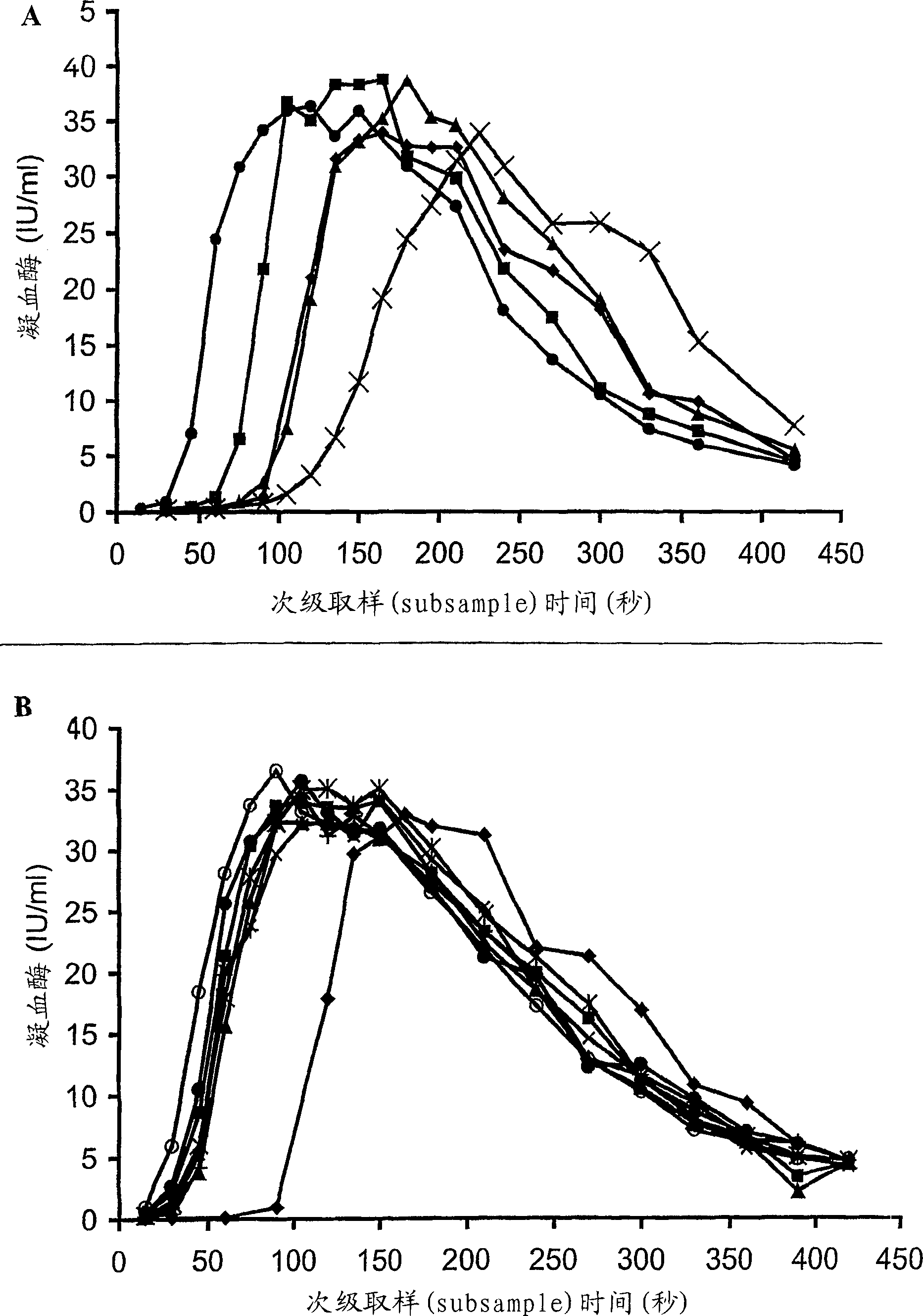
[0186] Dose response of TGT to FVIII
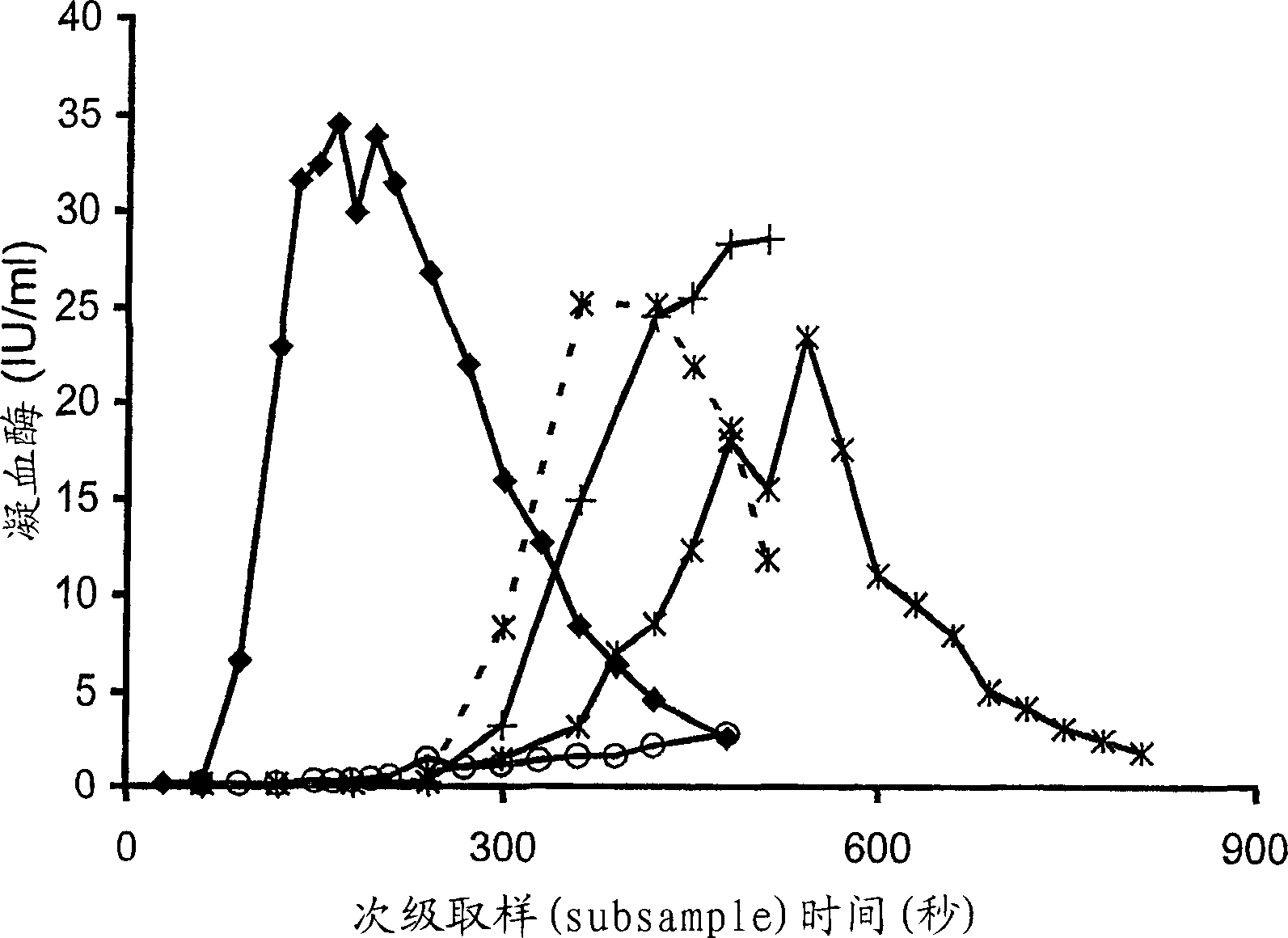
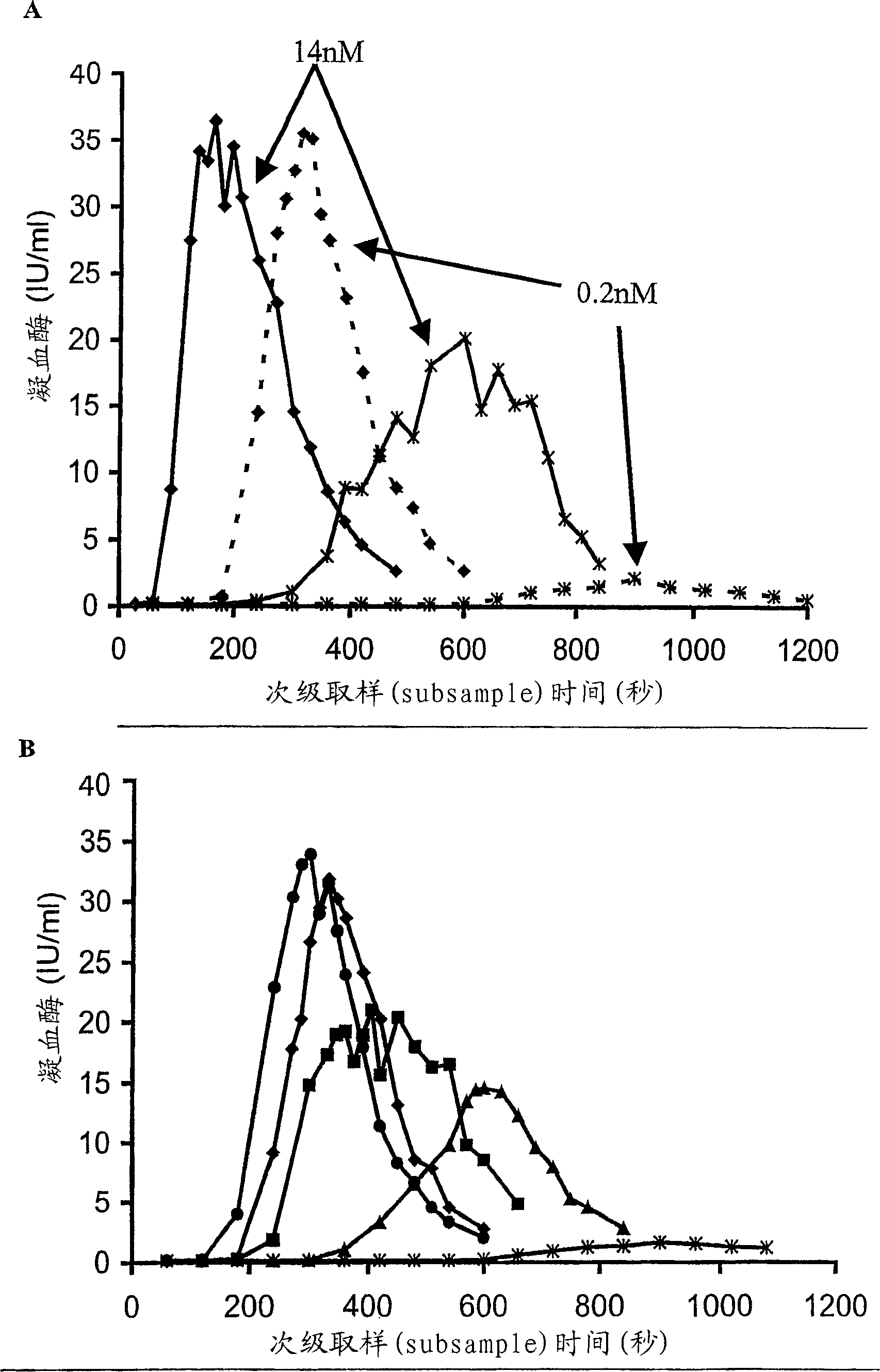
[0187] Concentrated HP(Mo-Ab)2 was spiked into artificially chemically depleted FVIII-deficient plasma with FVIII concentrations covering a wide range, from 0.005 to 1 IU / ml, and thrombin generation was measured (Fig 1A). When the concentration of FVIII was found to decrease from 1 to 0.005IU / ml, T1 / 2max The represented lag time was extended from 64 seconds to 165 seconds. However, despite very low levels of FVIII, peak thrombin was unaffected and only a small decrease in AUC was observed. Even when the concentration of FVIII was 0.005IU / ml, AUC was only slightly decreased compared with normal plasma, which were 6287 and 6540iu.sec, respectively. At a concentration of 1(T 1 / 2max 64sec) and 0.125IU / ml (T 1 / 2max 87sec), the ratio of thrombin to normal plasma (T 1 / 2max 118sec) produced faster, while the concentration of 0.03IU / ml (T 1 / 2max 119sec) was similar to that of normal plasma thrombin.
Embodiment 3
[0189] FVIII concentrate comparison
[0190] When different FVIII concentrates were added to FVIII-deficient plasma at the same FVIII:C concentration, the thrombin generation curves obtained were similar to those of the concentrate HP(Mo-Ab)2 ( figure 1 B, Table 2). All concentrates produced thrombin faster and in greater amounts than normal plasma at 1 IU / ml. T of rFVIII BDD 1 / 2max (48 sec) was shorter than the other concentrates (range 54-64 sec), this difference was statistically significant, p<0.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com