Protein for treatment of inflammatory diseases
An inflammatory disease and protein technology, applied in the field of lectin protein, can solve the problems of high demand, low output, and difficulty in buying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
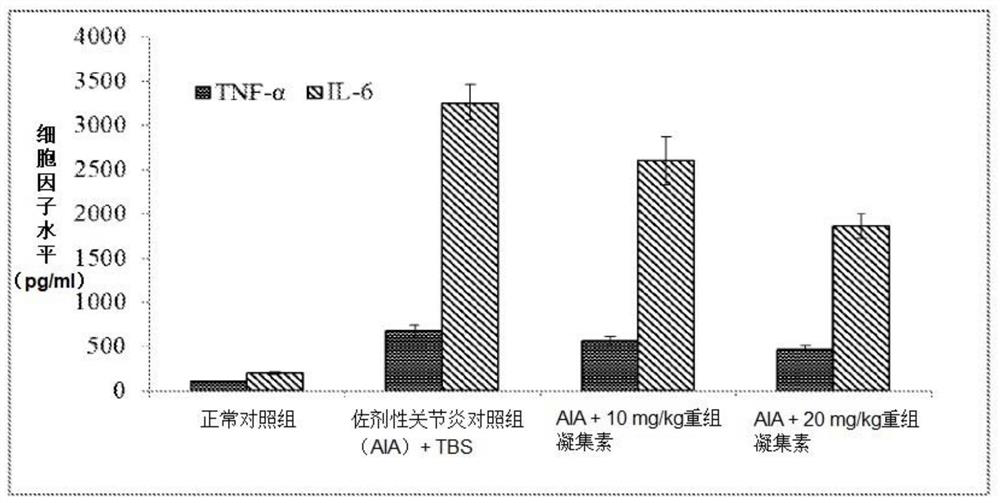
[0141] A study was carried out to evaluate the effect of a recombinant lectin having the amino acid sequence of SEQ ID NO: 2 on the secretion of inflammatory cytokines (IL-8 and IL-6) in a human synovial cell line (SW982). Recombinant lectins have the effect of inhibiting the secretion of inflammatory cytokines in cell lines.
[0142] Human synoviocytes (SW982) were first cultured for 24 hours, serum starved for 6 hours, and then the recombinant lectin with the amino acid sequence of SEQ ID NO: 2 and the inflammatory stimulus were used according to the non-toxic concentration of cells (1ng / ml-1,000ng / ml) (100 ng / ml IFN-γ) treated cells for 24 hours. The levels of cytokines (IL-6 and IL-8) in the supernatant were measured by ELISA. The inhibitory effect of recombinant lectins on cytokine secretion was calculated as follows:
[0143] Cytokine inhibition percentage=[(A-B) / A]*100
[0144] In the formula:
[0145] A = concentration of cytokines in control cells (IFN-γ only)
...
example 2
[0149] Another study was conducted to determine the effect of recombinant lectin having the amino acid sequence of SEQ ID NO: 2 on the secretion of inflammatory cytokines (TNF-α and The role of IL-1-β).
[0150] Spleen cells were isolated from C57BL / 6 mice and treated with recombinant lectin having the amino acid sequence of SEQ ID NO: 2 and lipopolysaccharide (LPS) at 25 ng / ml at a nontoxic concentration (1ng / ml-1,000ng / ml) 48 hours.
[0151] RAW264.7 cells were cultured for 24 hours, serum starved for 24 hours, and then the recombinant lectin with the amino acid sequence of SEQ ID NO: 2 and the inflammatory stimulus IFN-γ ( 100ng / ml) combined treatment of cells for 24 hours. The levels of cytokines (TNF-α and IL-1-β) in the supernatant were measured by ELISA.
[0152] The inhibitory effect of recombinant lectins on cytokine secretion was calculated as follows:
[0153] Cytokine inhibition percentage=[(A-B) / A]*100
[0154] In the formula:
[0155] A = concentration of c...
example 3
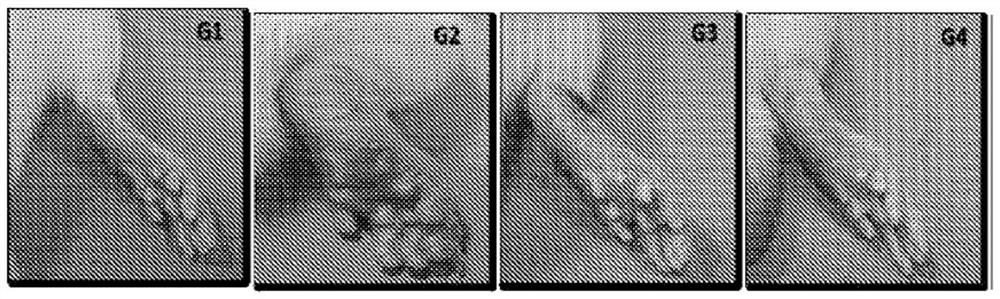
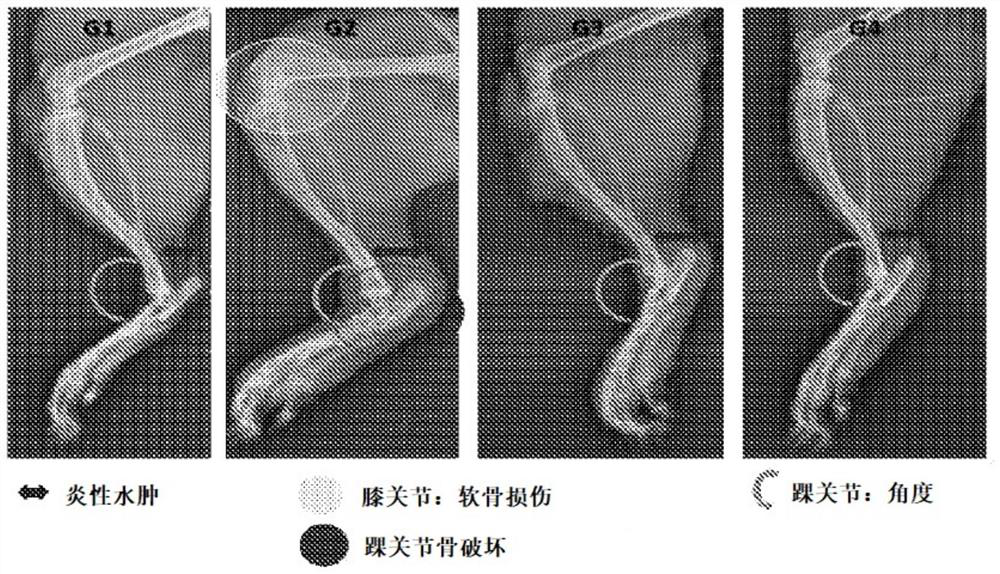
[0160] Another study was performed to determine the effect of a recombinant lectin having the amino acid sequence of SEQ ID NO: 2 on bone turnover markers (alkaline phosphatase (ALP) and collagen) in a human osteoblast cell line (MG-63). MG-63 cells were cultured for 24 hours, serum starved for 24 hours, and then treated with recombinant lectin having the amino acid sequence of SEQ ID NO: 2 at a nontoxic concentration (1 ng / ml-1,000 ng / ml) for 5 days. ALP activity in cell lysates was determined by the PnPP (p-nitrophenyl phosphate) method. Collagen levels in cell lysates were determined by Sircoll staining. The recombinant lectin having the amino acid sequence of SEQ ID NO: 2 can increase ALP activity (11%-42.8%) and collagen level (3.2%-45.3%) in a human osteoblast cell line (MG-63).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com