Modified factor ix polypeptides and uses thereof
A factor, D85I technology, applied in coagulation/fibrinolytic factors, medical preparations containing active ingredients, peptidase, etc., can solve problems such as coagulation defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1: Cloning of Human Factor IX cDNA
[0138] A pair of PCR primers complementary to the libraries at the 5' and 3' ends of the human FIX cDNA coding region were designed according to the published cDNA sequence (NM_000133). 5' primer (FIXF1; ATCATAAGCTT GCCACC ATGCAGCGCGTGAACATG (SEQ ID NO: 3), FIX start codon in bold) contains the first 18 nucleotides of the FIX coding region, including the ATG start codon and HindIII following the consensus Kozak sequence (underlined) restriction site. The 3' primer (FIXR3, ATCATAAGCTTGATTAGTTAGTGAGAGGCCCTG) (SEQ ID NO: 4) contained 22 nucleotides of the FIX sequence located 3'45 nucleotides from the end of the FIX coding region, preceded by a HindIII site. Amplification of cDNA first strand from normal human liver (Stratagene, San Diego, CA) using these primers and a high-fidelity proofreading polymerase (Invitrogen, Carlsbad, CA) resulted in a single band of the expected size (1464 bp) for human FIX cDNA. After digestion ...
Embodiment 2
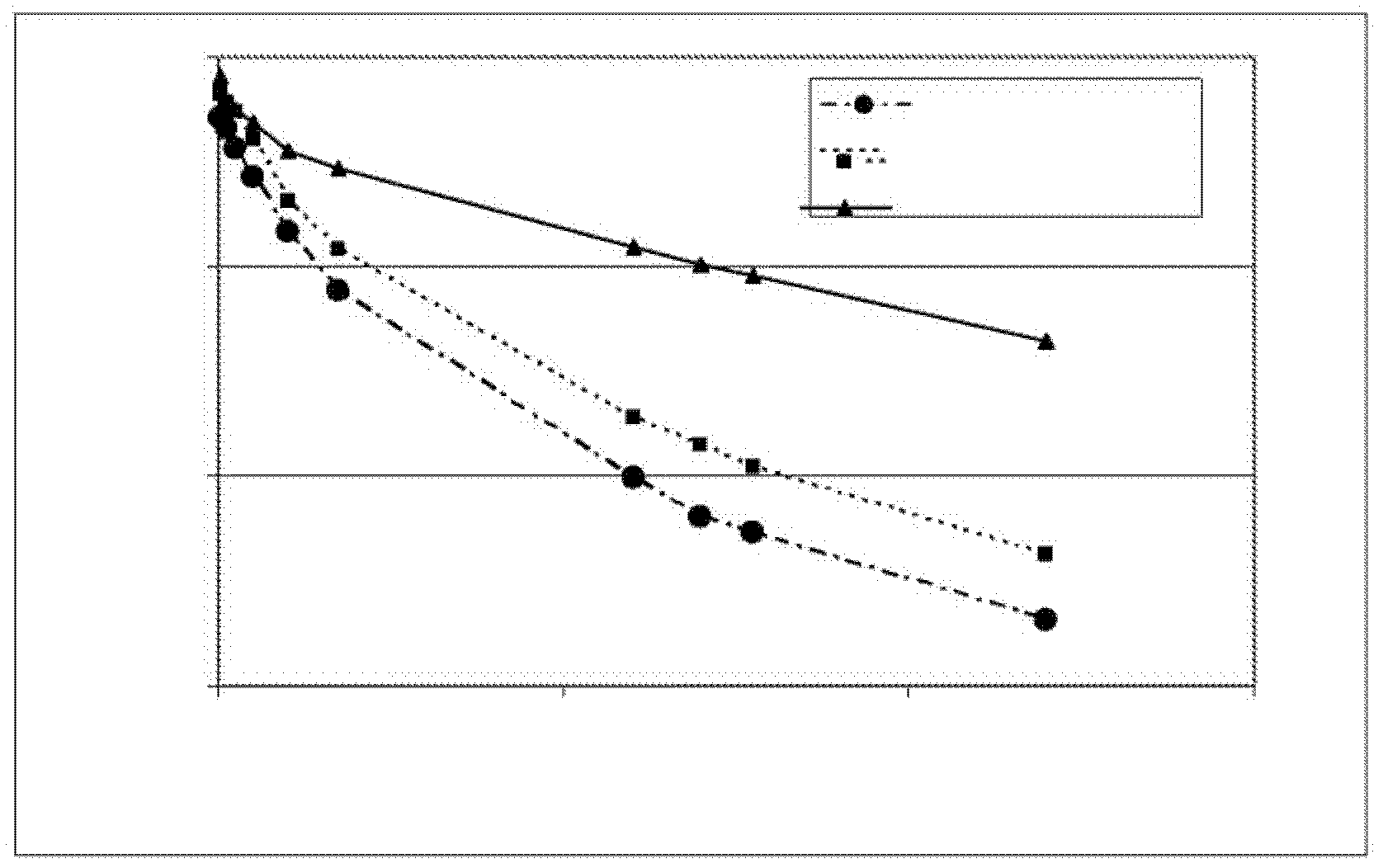
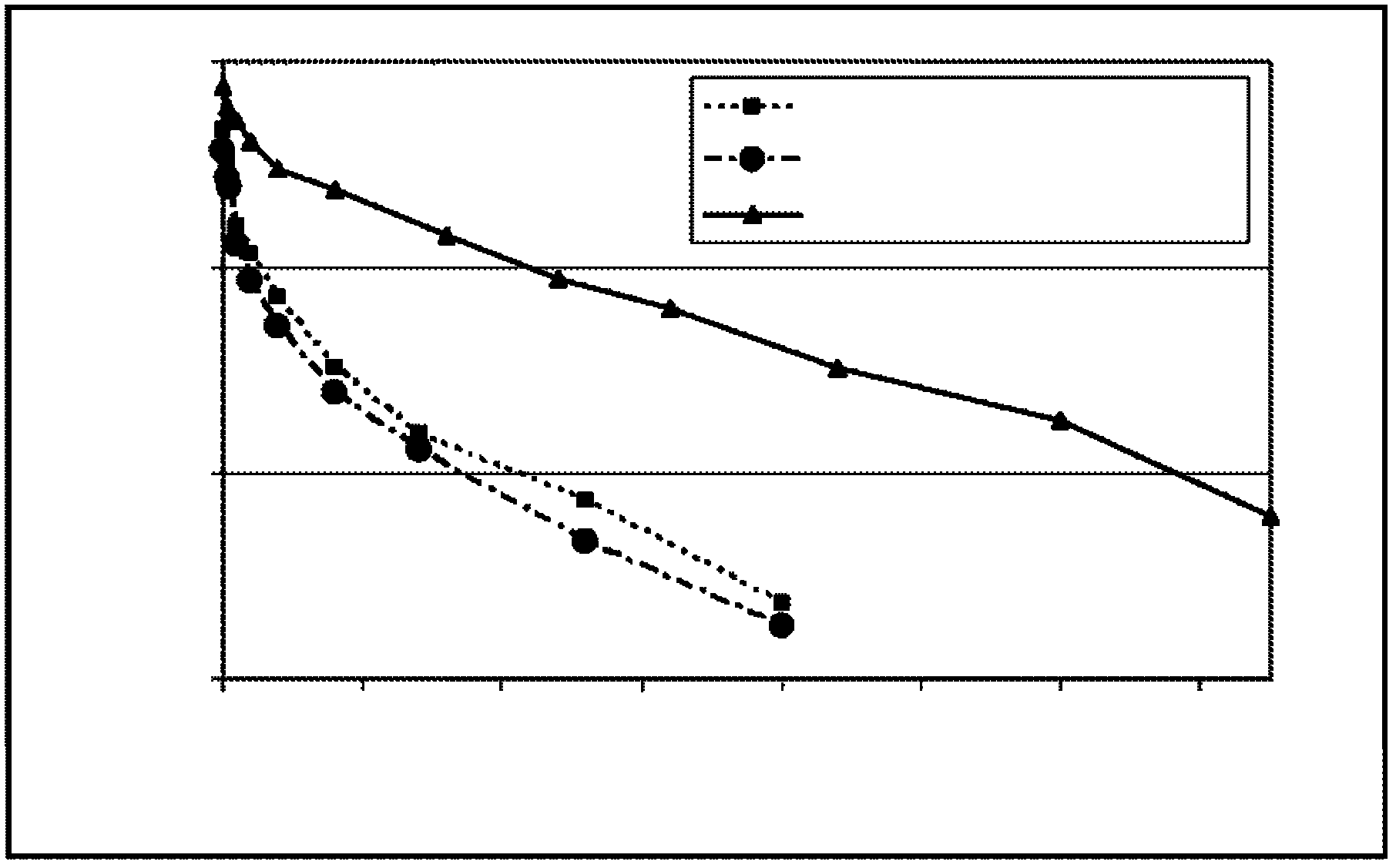
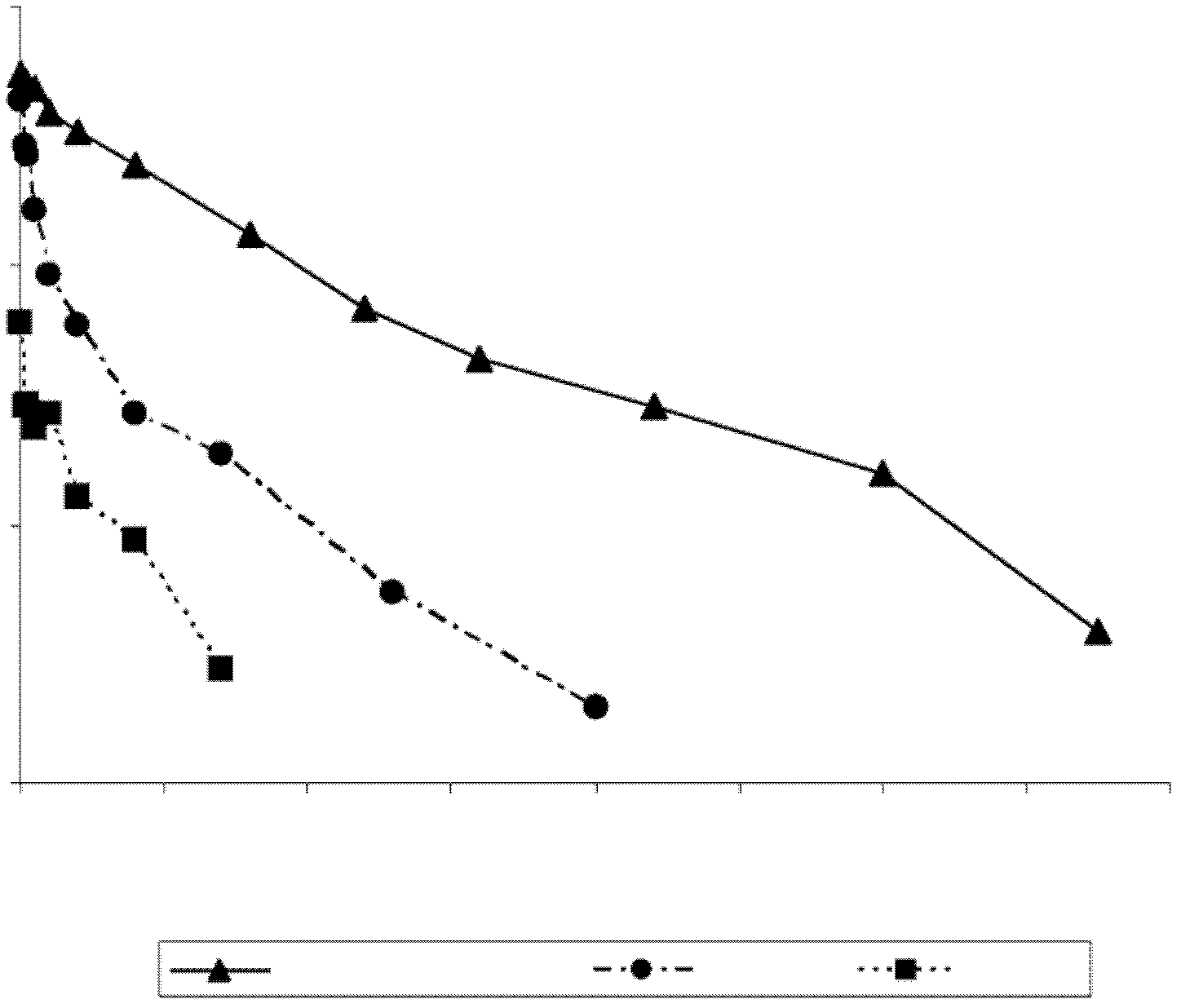
[0139] Example 2: Generation of Modified Factor IX Polypeptides
[0140] To change individual amino acids within the human FIX sequence, use the Quickchange TM A primer design program (Stratagene, San Diego, CA) designed a pair of primers. Adopt Quickchange TM The II XL Site-Directed Mutagenesis Kit (Stratagene, San Diego, CA) used these primers to generate mutations in the pEAKflcmv-FIX plasmid according to the manufacturer's instructions. Clones containing the desired mutation were identified by DNA sequencing of the entire FIX coding region. The sequences of the sense strand oligonucleotides used to create the mutations are shown in Table 1.
[0141] Table 1
[0142]
[0143]
[0144]
[0145]
[0146]
Embodiment 3
[0147] Example 3: Expression of Factor IX Polypeptide in HKB11 Cells
[0148] To determine whether FIX genes with altered protein sequences could be expressed and secreted from mammalian cells and to determine the effect of these substitutions on FIX clotting activity, expression plasmids encoding these FIX variants were transfected into HKB11 cells. HKB11 is a human cell line generated by fusion of HEK293 cells and B-cell lymphoma.
[0149] HKB11 cells were grown in suspension culture on an orbital shaker (100-125rpm) in CO 2 (5%) cultured in a serum-free medium (Sigma-Aldrich, St.Louis, MO) supplemented with 10ng / mL soluble vitamin K3 at 37°C in an incubator and maintained between 0.25 and 1.5x10 6 The density between cells / mL.
[0150] Cells for transfection were harvested by centrifugation at 1000 rpm for 5 minutes and then placed in a FreeStyle TM 293 expression medium (Invitrogen, Carlsbad, CA) with 1.1x 10 6 cells / mL resuspended. Cells were seeded in 6-well plates ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com