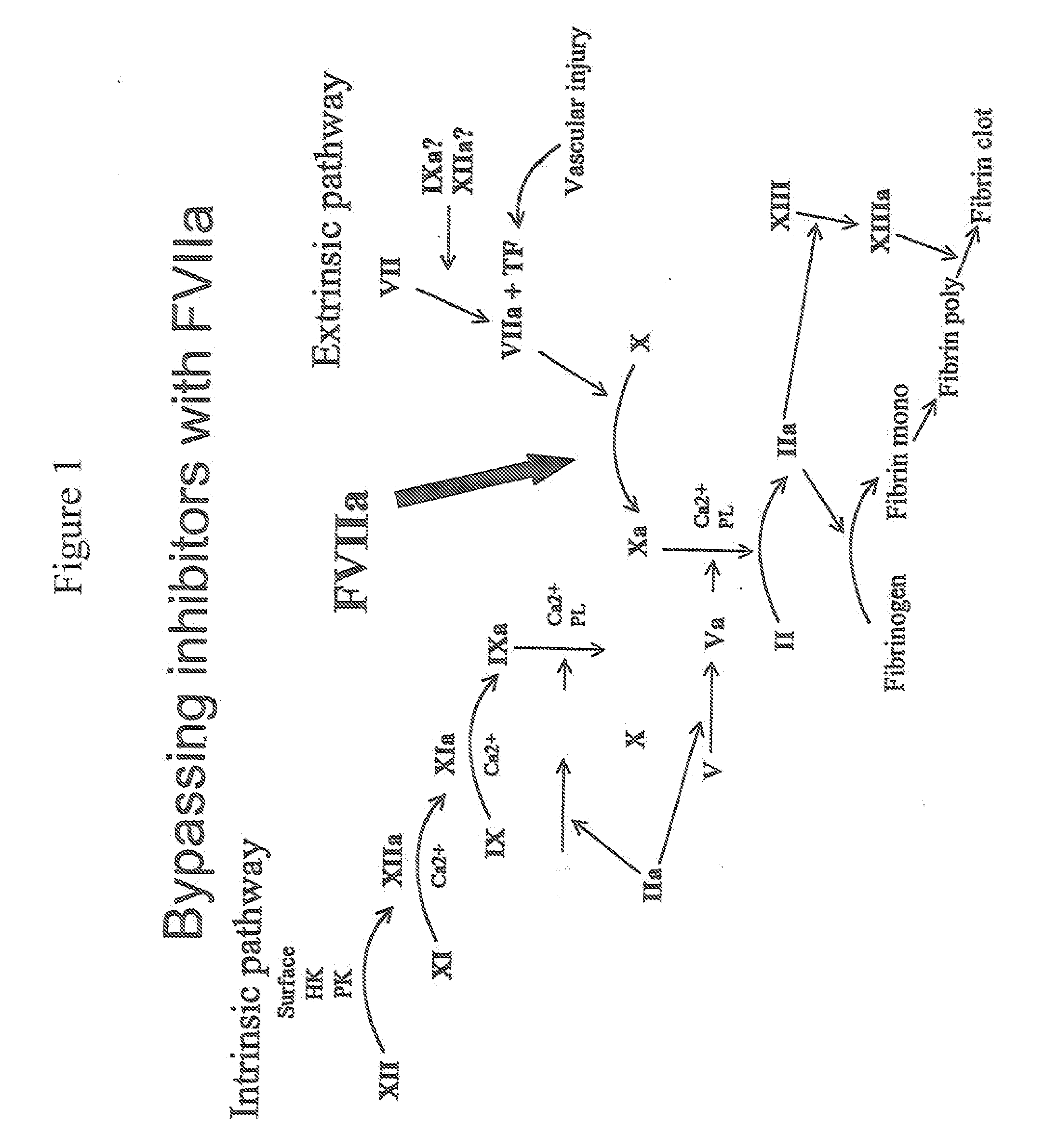
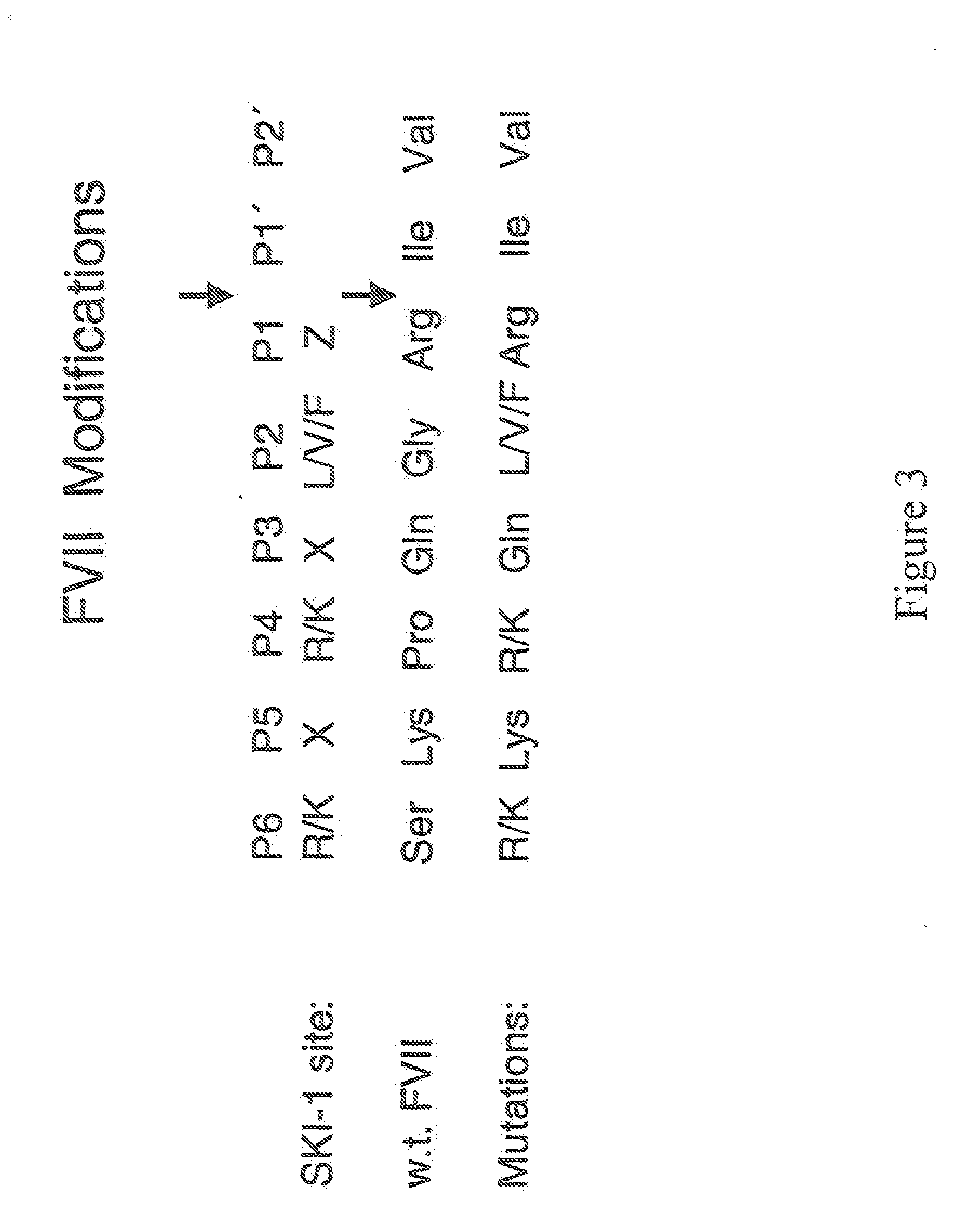
Methods for treating blood coagulation disorders
a blood coagulation disorder and coagulation method technology, applied in the field of xlinked bleeding disorder, can solve the problems of ineffective protein replacement or gene replacement therapy, intrinsic blood clotting pathway involving fviii and fixation, and high cost of recombinant fviia manufacture, so as to achieve the effect of optimally promoting proper disulfide bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Materials & Methods
Cloning of FVII:
[0085]The factor VII cDNA was cloned by PCR (Perkin Elmer, 25 cycles) from a human liver cDNA library (Clontech) using primer 5432JS (5′-CTAGCCTAGG CCACCATGGT CTCCCAGGCC CTCAGGCTC-3′ and primer 5433JS (5′-CCTTAATTAA CTAGGGAAAT GGGGCTCGCA GGAG-3′. The PCR product was cloned into a pCR-Blunt-II TOPO vector (Invitrogen), sequenced for accuracy and then subcloned into pCMV expression vector, which has the CMV promoter / enhancer and an SV40 poly A.
Cloning of FVII Light Chain (LC):
[0086]The FVII light chain was cloned by PCR from the plasmid pCMV / hFVII using primer 5432JS shown above and primer 5479JS (5′-GCTAGCCTAT CGGCCTTGGG G-3′). This construct contains the FVII leader sequence and amino acids #1 (Ala) to #152 (Arg). The PCR product has been cloned into the pCR-Blunt-II TOPO vector, sequenced for accuracy and then subloned into the pCMV expression vector.
Cloning of FVII Heavy Chain (HC):
[0087]The FVII heavy chain was cloned by three primer PCR from th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com