Pharmaceutical Composition Comprising Factor VII Polypeptides and TAFI Polypeptides
a technology of factor vii and polypeptides, which is applied in the direction of drug compositions, peptide/protein ingredients, extracellular fluid disorder, etc., can solve the problems of complex structure, high cost, and high risk of human virus transmission, and achieve the effects of improving coagulation, reliable and widely applicable, and enhancing or ensuring formation of stable haemostatic plugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Improving Haemostatic Clot Stability by Combining Coagulation Factors Vlla and TAFI
Methods:
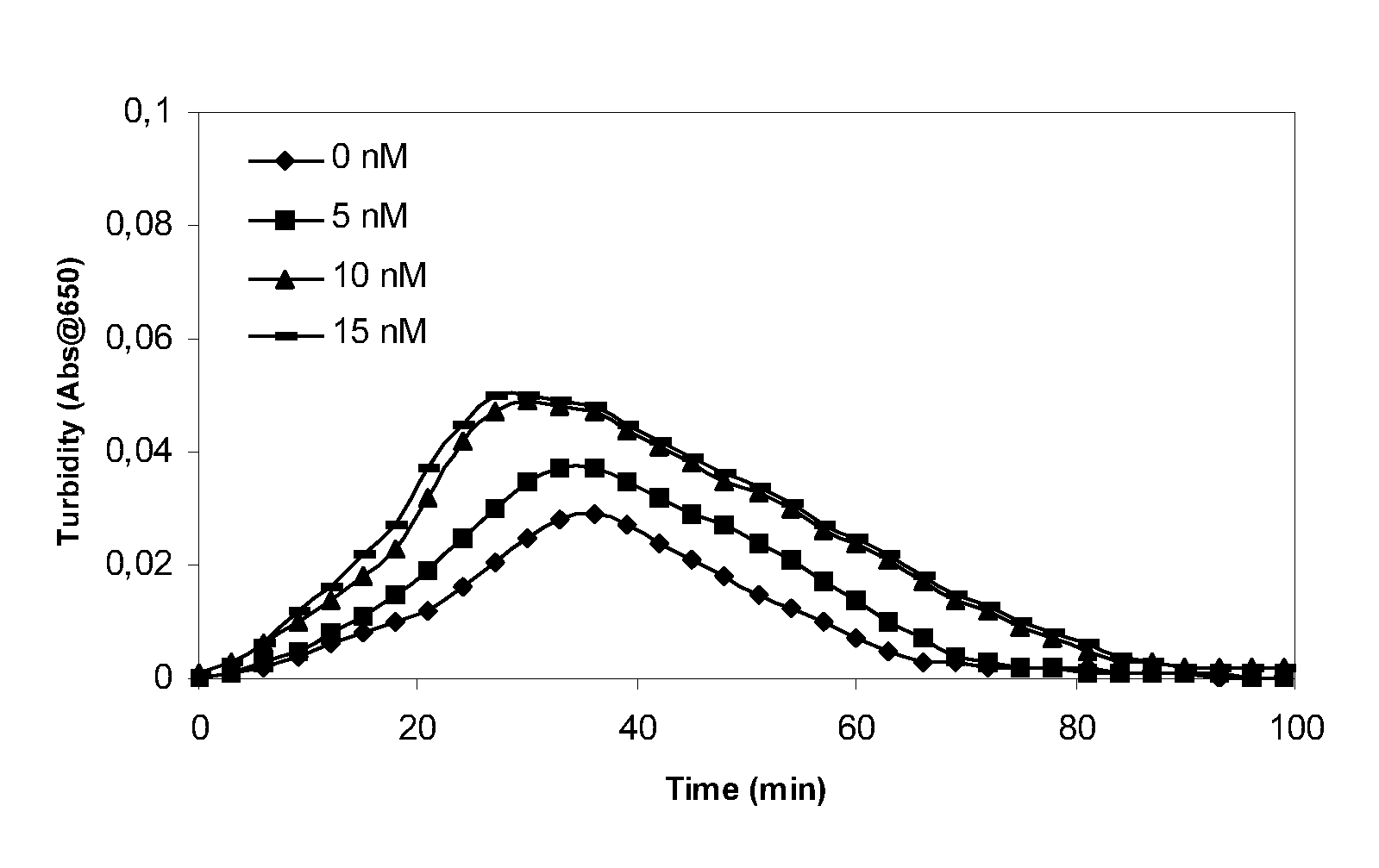
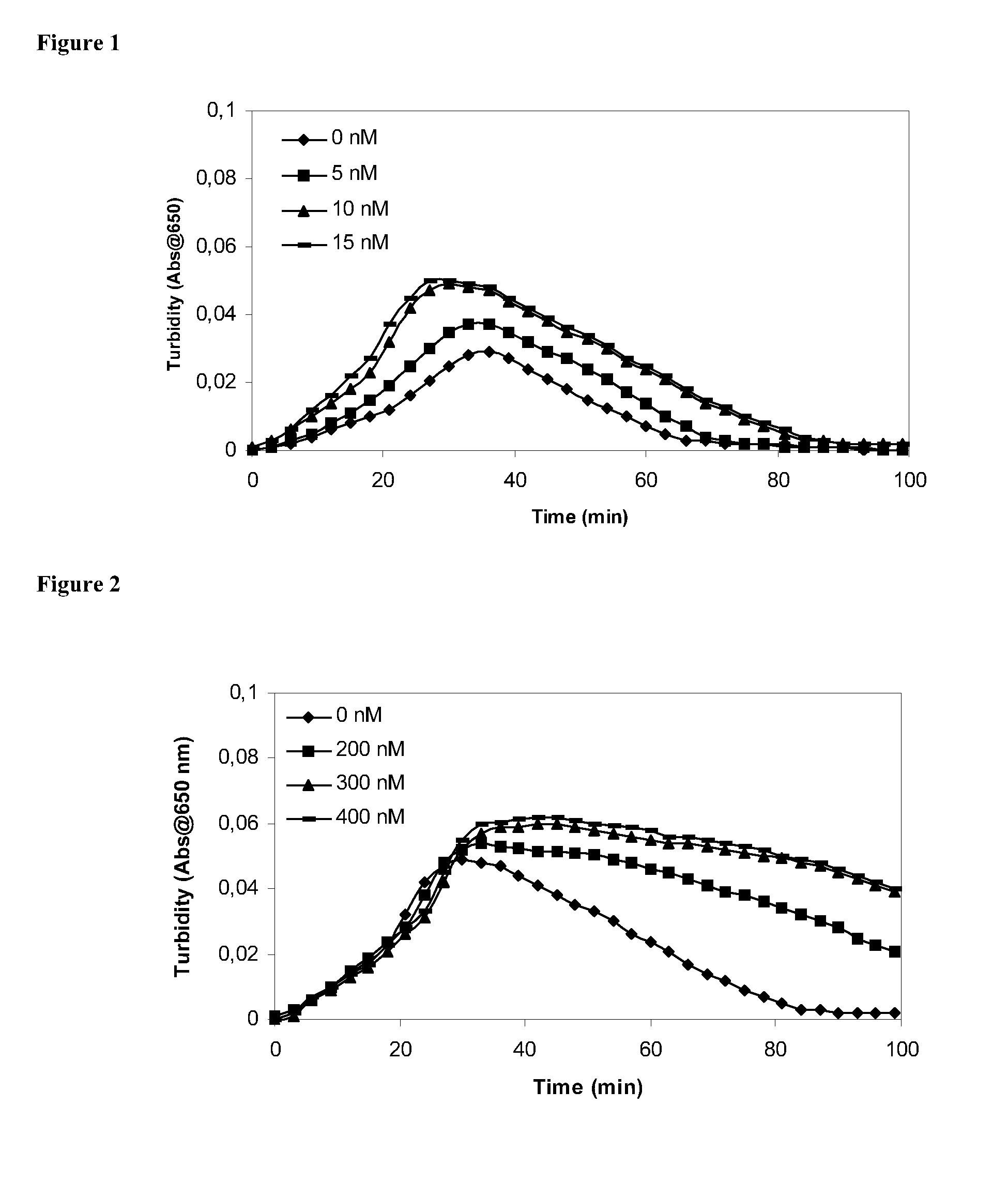
[0205] Clot lysis assay: Normal human plasma diluted 10-fold with buffer (20 mM HEPES, 150 mM NaCl, 5 mM CaCl, pH 7.4) containing Innovin (Dade Behring, 2000-fold dilution), rFVIIa (Novo Nordisk A / S, Bagsvaerd, Denmark; various concentrations) and t-PA (American Diagnostics, 8 nM) was added to 96-well ELISA plates and turbidity at 650 nm was measured over time at room temperature. Where indicated, purified human TAFI (Bajzar et al., J. Bioac Chem. 270:14477, 1995, various concentrations) was included.
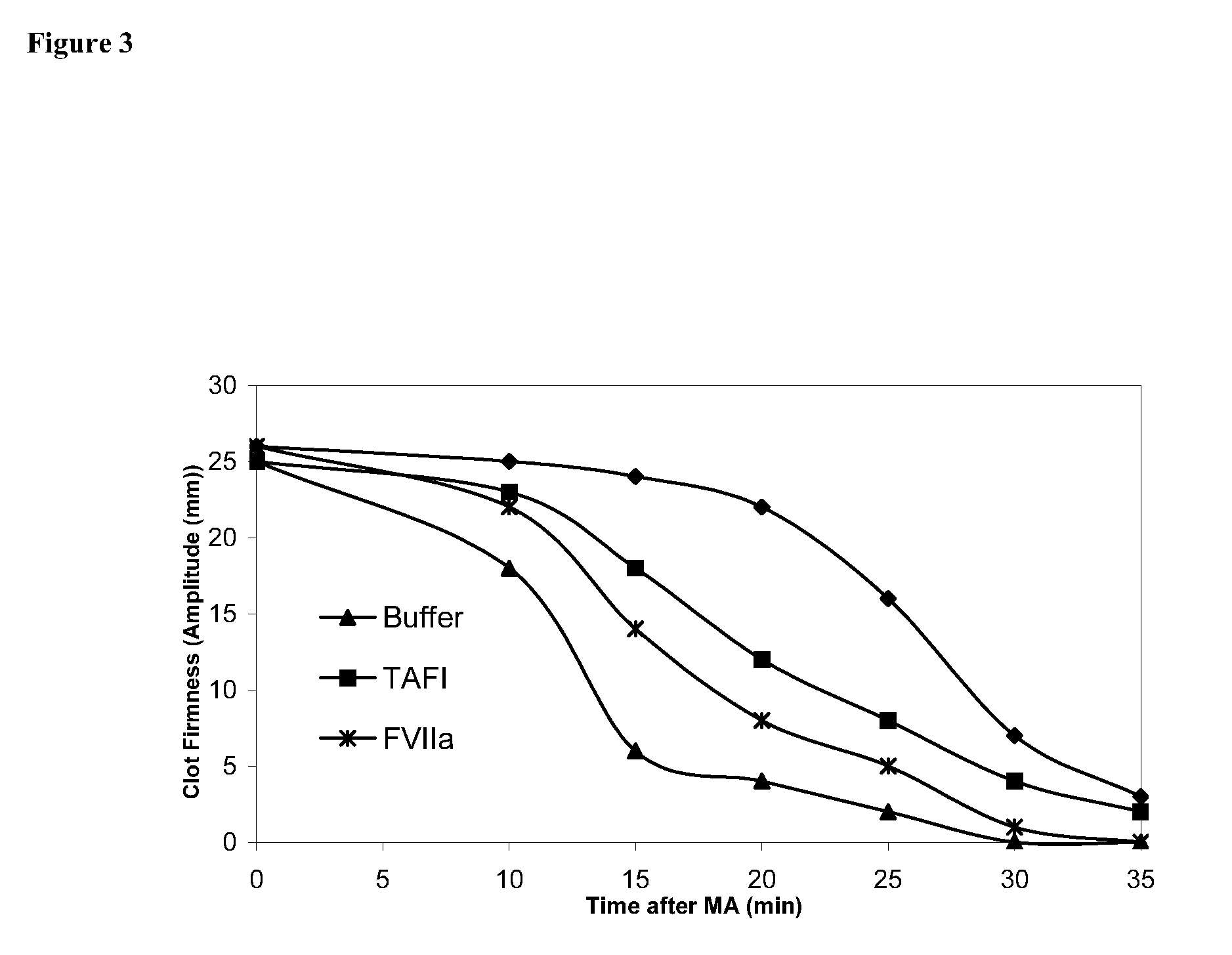
[0206] Rotatonal thromboelastography (roTEG): Measurements was conducted on citrated normal human plasma added 5 nM t-PA and the effect of addition of 1 nM FVIIa alone or in combination with 30 nM TAFI was analyzed. Clotting was initiated by addition of Innovin (final concentration 2000-fold diluted, Dade Behring #526945) and calcium (final concentration 15 mM) in a 20 mM HEPES, 150 mM NaCl, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com