Pegylated factor VII glycoforms
a technology of glycoforms and pegylated factor, which is applied in the field of compositions, can solve the problems of difficult to obtain adequate dose regulation and restrict the patient's way of living, and achieve the effect of improving functional properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Glycopegylation of Product with High Sialic Content
[0183] Polyethylene glycol-CMP-sialic acid (PEG-CMPSA) is prepared by covalently attaching PEG with mw 10.000 Da to sialic acid.
[0184] Factor VIIa with 87-99% content of sialic acid is treated with sialidase, e.g., as described in U.S. Pat. No. 5,272,066, and re-sialylated with sialyltransferase using PEG-CMPSA as donor molecule (e.g., as described in U.S. Pat. No. 6,399,336). After the PEGylation reaction has reached maximal incorporation, CMPSA is added to the reaction mixture to cap any exposed terminal galactose.
[0185] Incorporation of PEGylated sialic acid is analyzed by SDS-PAGE, CE-PAGE, isoelectric focusing gels, and CE-IEF.
[0186] 94-100% sialic acid is incorporated; a mean of 1-4 PEG groups are incorporated.
example 2
Glycopegylation of Product with Medium Sialic Content
[0187] Polyethylene glycol-CMP sialic acid (PEG-CMPSA) is prepared by covalently attaching PEG with mw 10.000 Da to sialic acid.
[0188] Factor VIIa with 87-93% content of sialic acid is treated with sialyltransferase using PEG-CMPSA as donor molecule (e.g., as described in U.S. Pat. No. 6,399,336). After the PEGylation reaction has reached maximal incorporation, CMPSA is added to the reaction mixture to cap any exposed terminal galactose.
[0189] Incorporation of PEGylated sialic acid is analyzed by SDS-PAGE, CE-PAGE, isoelectric focusing gels, and CE-IEF.
[0190] 87-100% sialic acid is incorporated; a mean of 0.1-0.5 PEG groups are incorporated.
example 3
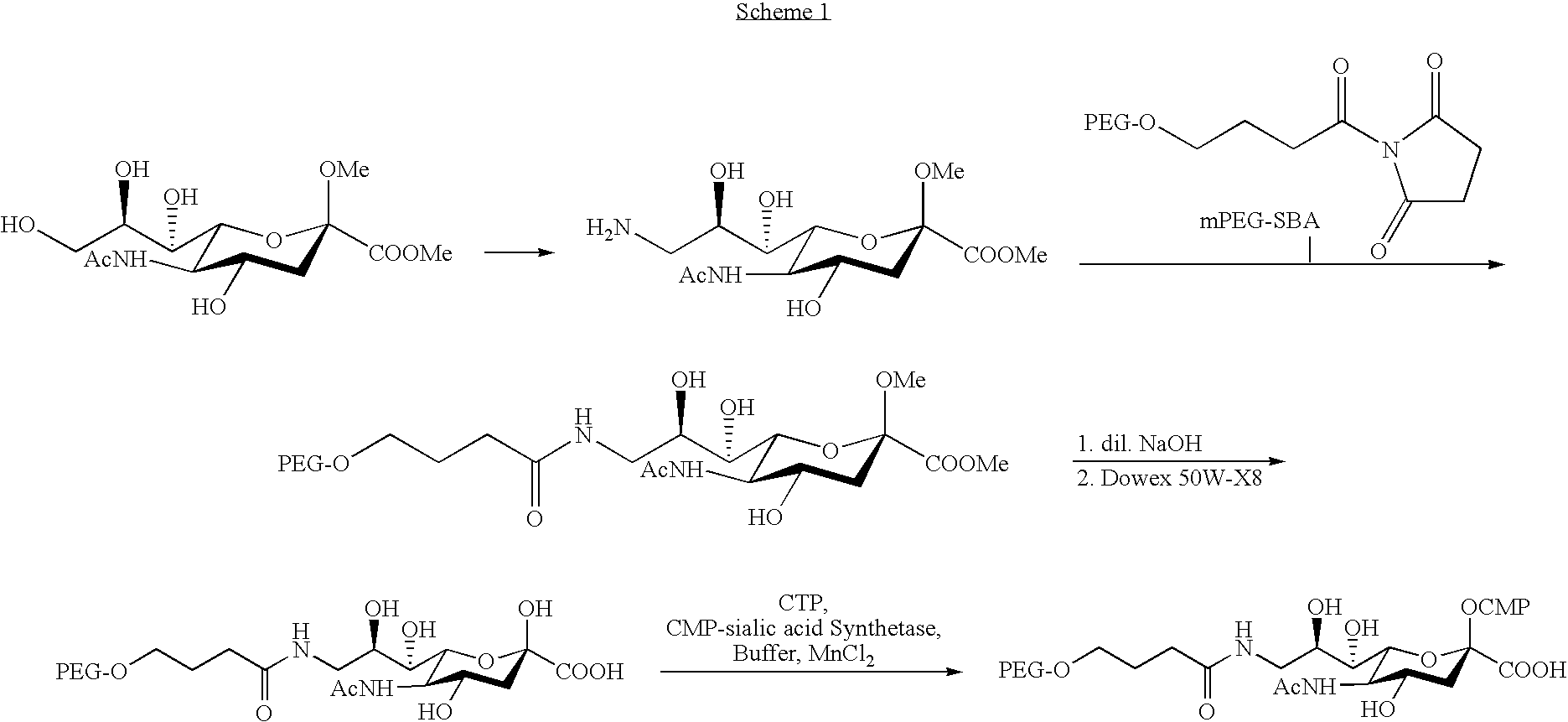
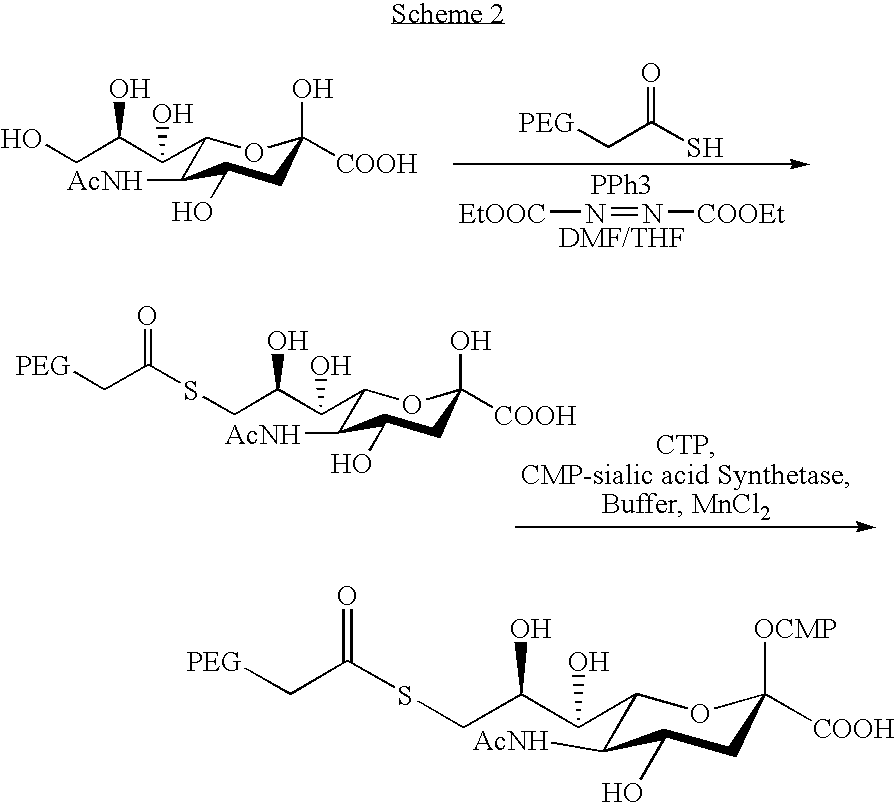
[0191] Pegylated cytidine 5′-monophospho-sialic acid derivative (CMP-SA-PEG): N-Acetyl-O2-methyl-9-amino-9-deoxy-neuraminic acid methyl ester (10 mg, 0.031 mmol, prepared according to Isecke, R.; Brossmer, R., Tetrahedron 1994, 50(25), 7445-7460) is dissolved in water (2 ml), and mPEG-SBA (170 mg, 0.03 mmol, 5 kDa, Shearwater 2M450H01)) is added. The mixture is stirred at ambient temperature until completion according to TLC. The solvent is removed by lyophilization, and the residue redissolved in a 1:1 mixture of methanol and 0.1 M NaOH solution (5 ml). The mixture is stirred at room temperature for 1 h, then passed through a column of Dowex 50W-X8 (H+) resin at 4° C. and lyophilized. The residue is then redissolved in water (5 ml), Dowex 50W-X8 (H+) resin is added and the mixture is stirred until completed by TLC.
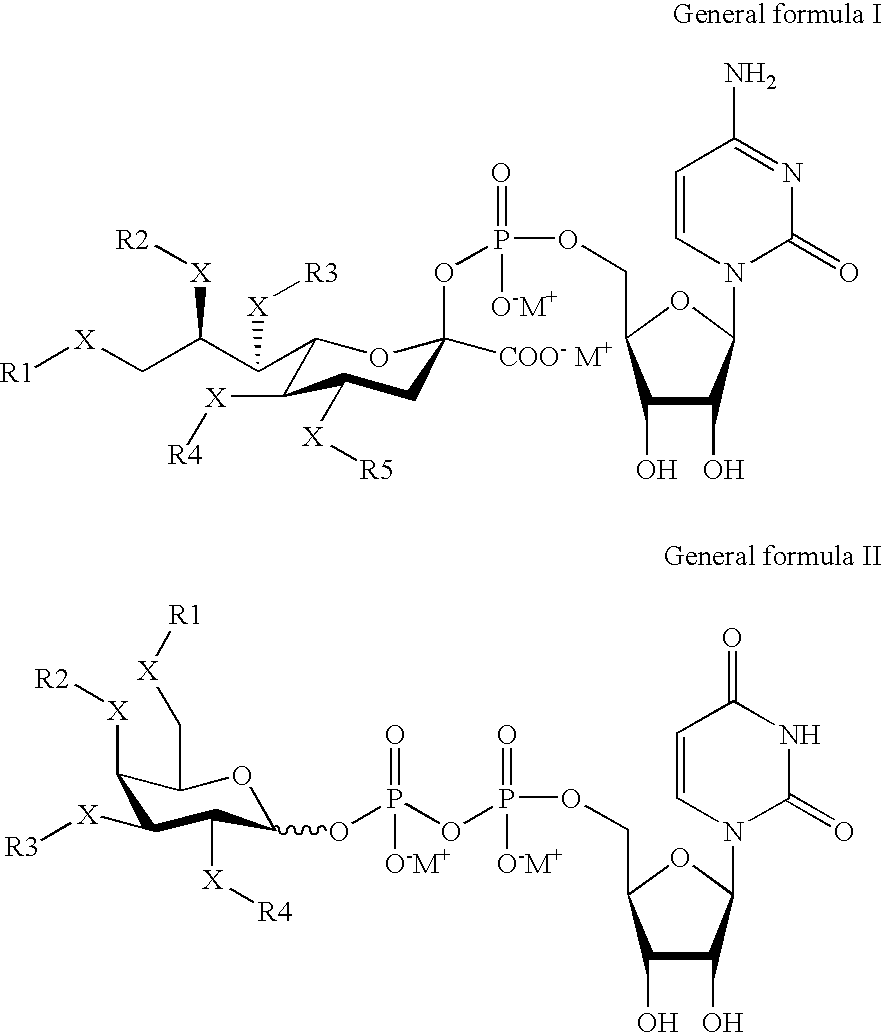
[0192] Cytidine 5′-monophospho analogues of sialic acid derivatives of general formula I is in general prepared according to E. S. Simon, M. D. Bednarski and G. M. White...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com