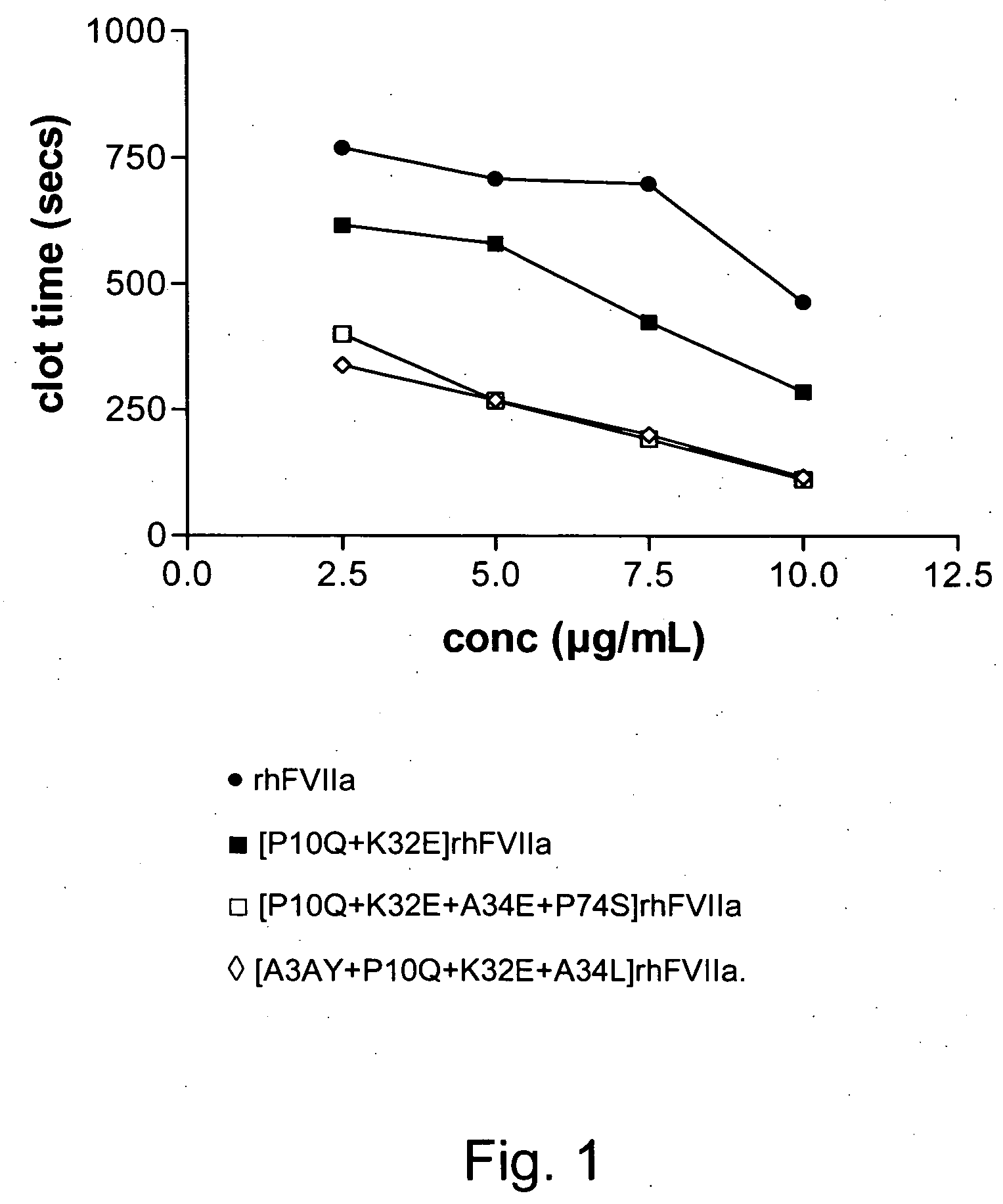
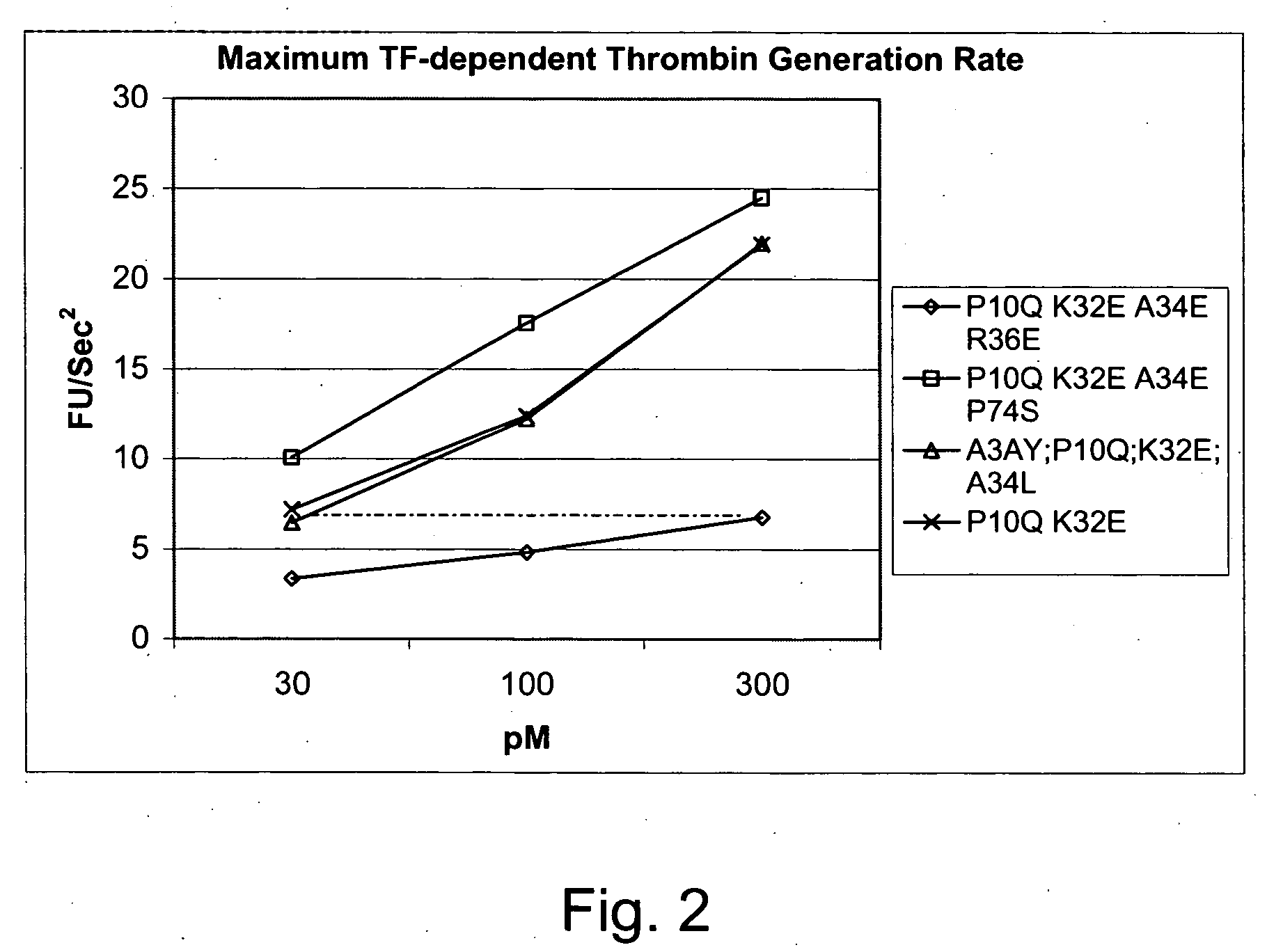
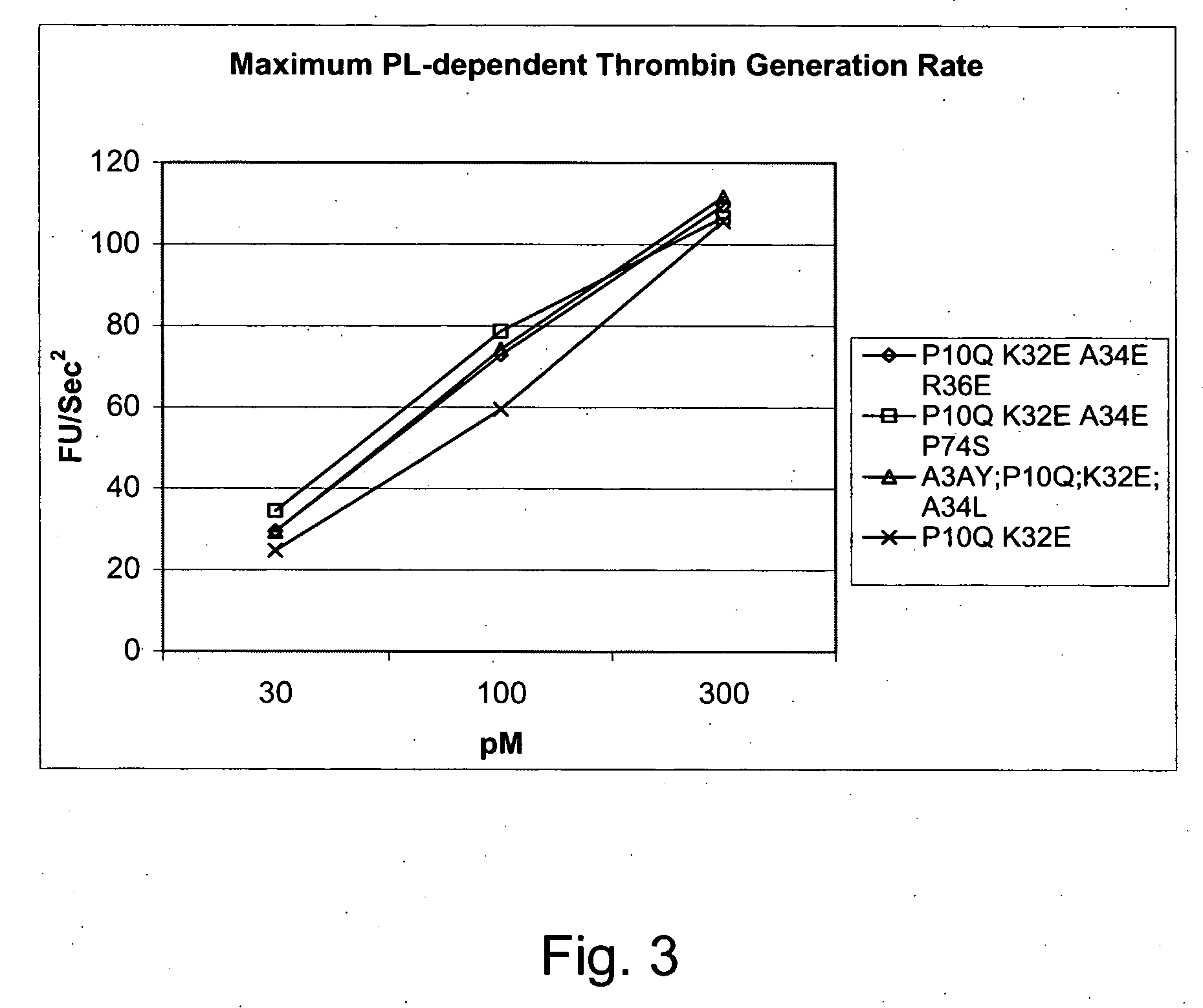
FVII or FVIIa Gla domain variants
a gla domain and variant technology, applied in the field of gla domain variants, can solve the problems of treatment being associated with a non-significant increase in thromboembolic events, and achieve the effect of reducing the number of thromboembolic events
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0257] The X-ray structure of hFVIIa in complex with soluble tissue factor by Banner et al., J Mol Biol, 1996; 285:2089 is used for this example. For further information on the calculations in this example, see WO 01 / 58935.
Surface Exposure
[0258] Performing fractional ASA calculations resulted in the following residues being determined to have more than 25% of their side chain exposed to the surface: A1, N2, A3, F4, L5,E6,E7,L8,R9,P10,S12,L13,E14,E16,K18,E19,E20,Q21, S23,F24, E25, E26, R28, E29, F31, K32, D33, A34, E35, R36, K38, L39, W41, I42, S43, S45, G47, D48, Q49, A51, S52, S53, Q56, G58, S60, K62, D63, Q64, L65, Q66, S67, I69, F71, L73, P74, A75, E77, G78, R79, E82, T83, H84, K85, D86, D87, Q88, L89, I90, V92, N93, E94, G97, E99, S103, D104, H105, T106, G107, T108, K109, S111, R113, E116, G117, S119, L120, L121, A122, D123, G124, V125, S126, T128, P129, T130, V131, E132, I140, L141, E142, K143, R144, N145, A146, S147, K148, P149, Q150, G151, R152, G155, K157, V158, P160, K16...
example 2
Design of an Expression Cassette for Expression of rhFVII in Mammalian Cells
[0263] The expression cassette for expression of rhFVII was designed and cloned as described in Example 2 of WO 01 / 58935.
example 3
Construction of Expression Cassette Encoding Variants of the Invention
[0264] Sequence overhang extension (SOE) PCR was used for generating constructs having variant FVII open reading frames with substituted codons by using standard methods.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com