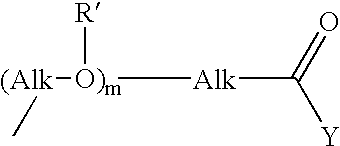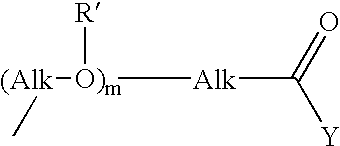Patents
Literature
115 results about "Cytostatic agents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytostatic Agent. a drug that blocks cell division. The mechanism by which cytostatic agents suppress certain stages of cell division vary. For example, alkylating agents, such as embichin (mechlorethamine hydrochloride) and cyclophosphamide, react directly with DNA.
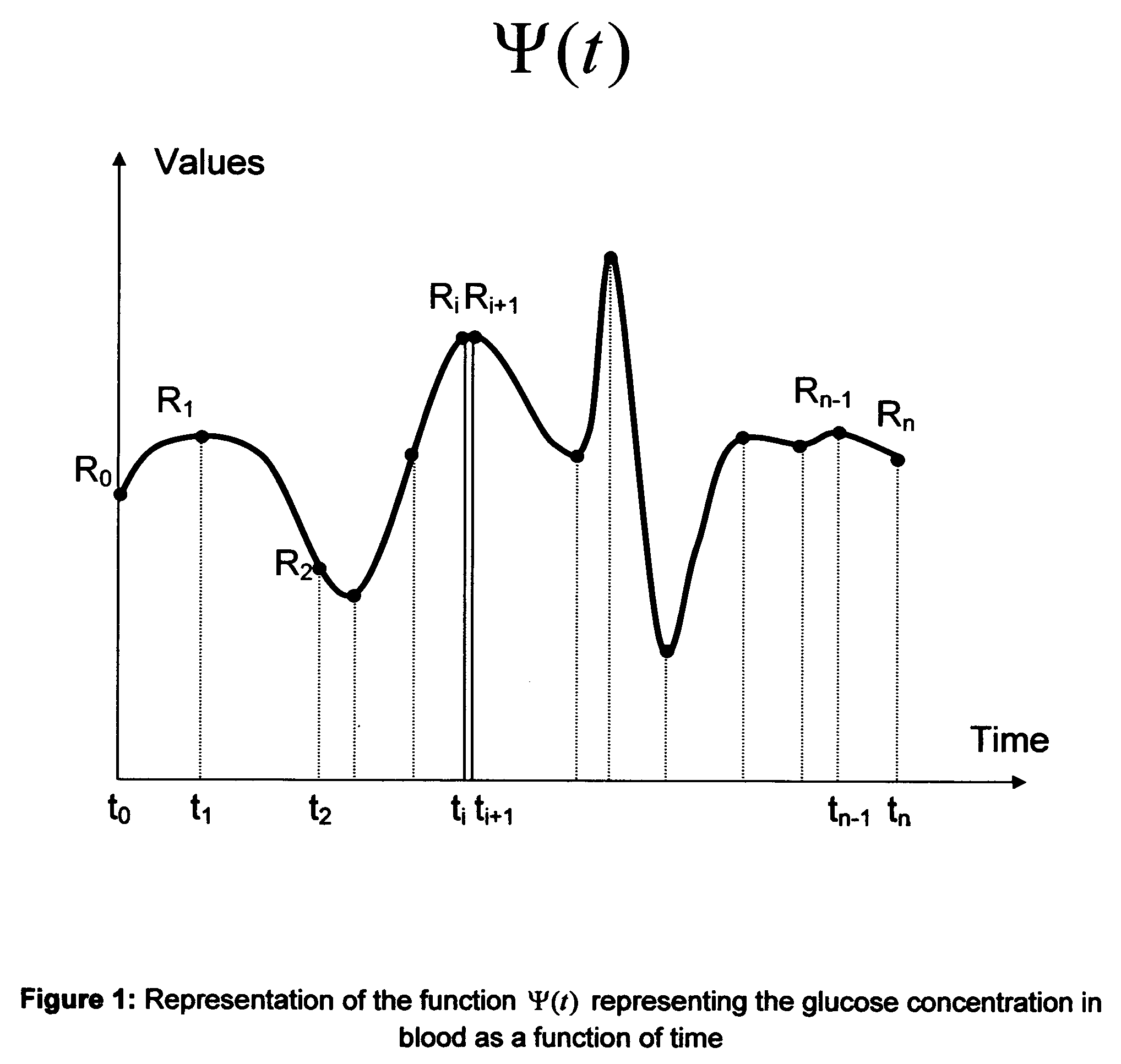
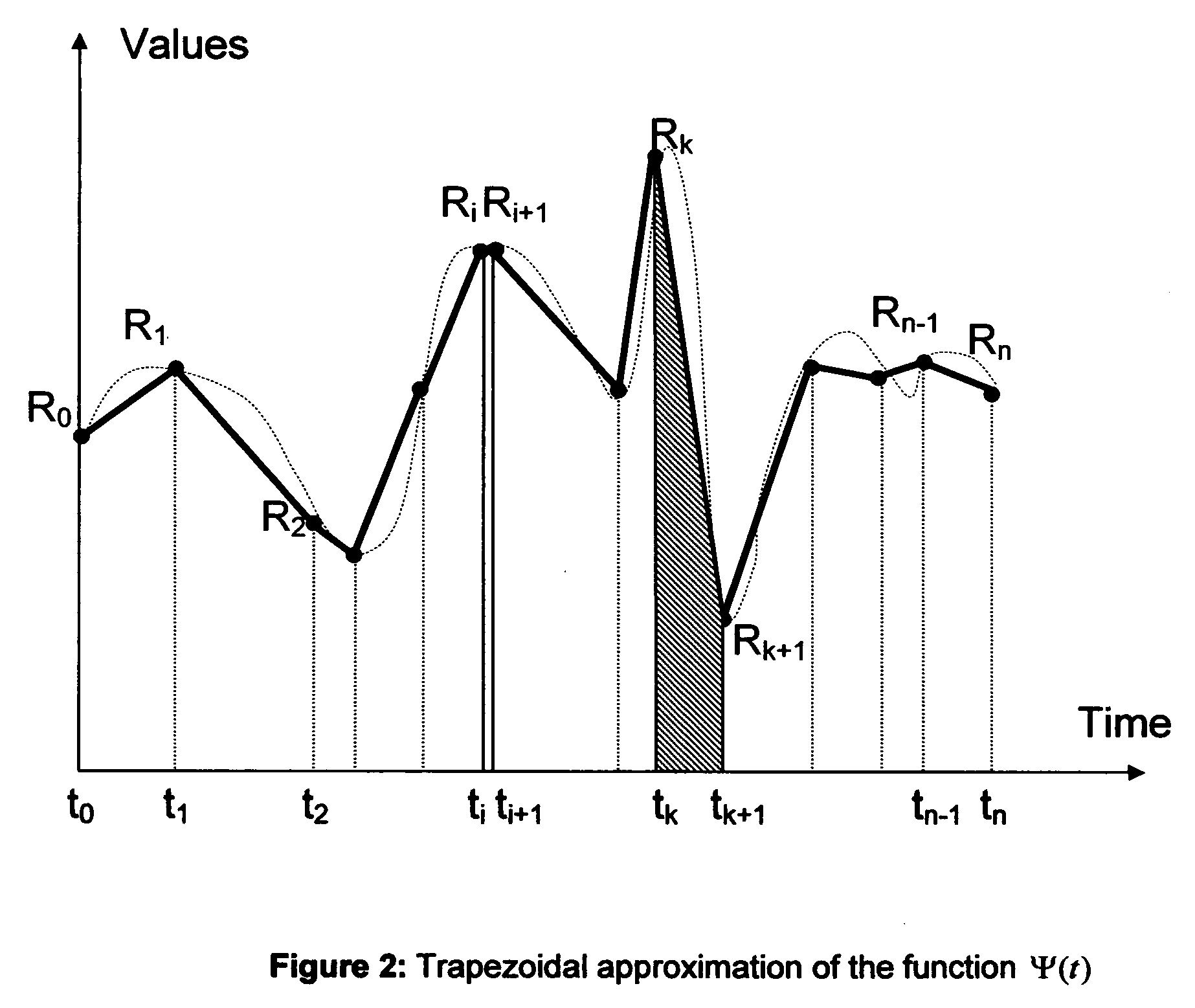
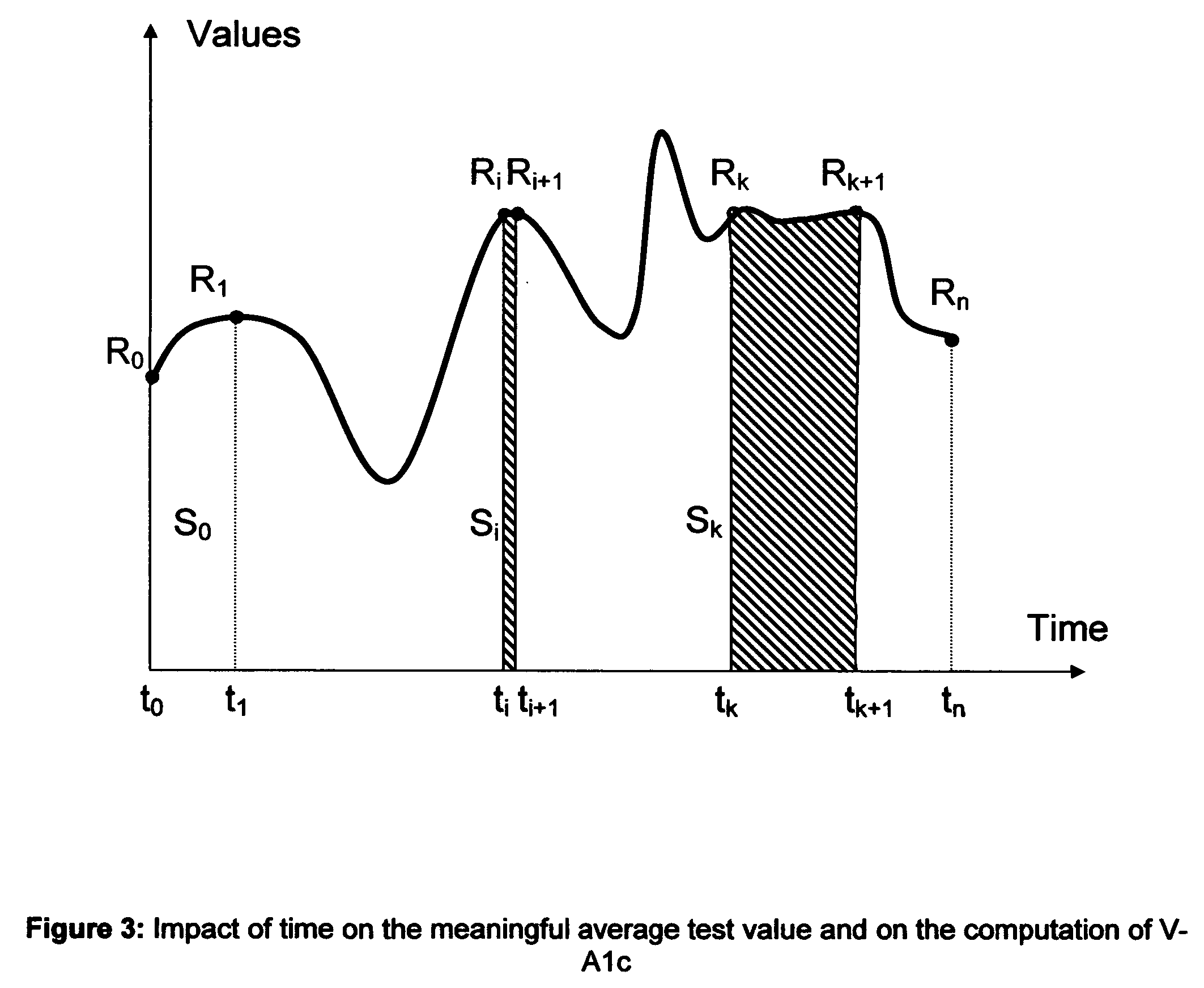
Method to determine the degree and stability of blood glucose control in patients with diabetes mellitus via the creation and continuous update of new statistical indicators in blood glucose monitors or free standing computers
InactiveUS20070010950A1Increase contributionShorten the timeDiagnostic recording/measuringSensorsInstabilityGlucose control
Microvascular complications of diabetes mellitus are closely related to blood glucose levels and fluctuations. The Glycostator statistical package was created to allow patients and health care providers simple access to “glycemic indicators” which permit a “snapshot view” of the effectiveness of the patient's diabetes management program. Glycostator functions provide a simple way of enhancing the information already provided by home blood glucose monitoring devices. To this end, a set of new indices, including one called the Virtual A1c, are computed in a recursive fashion from blood glucose test results to provide a more meaningful day-to-day assessment of glycemic control. All indices can be made available at the meter user interface on request. The displayed indices allow patients to improve glycemic control by identifying problems with blood glucose control and lability that are less easily recognized in traditional blood glucose meter statistical packages. Virtual A1c emulates hemoglobin A1c continuously and provides better day-to-day assessment of long term glycemic control than does the traditional average blood glucose report. The method for computing each of these indices, including the Virtual A1c, allows for their implementation in commercial blood glucose monitors.
Owner:ROCHE DIABETES CARE INC
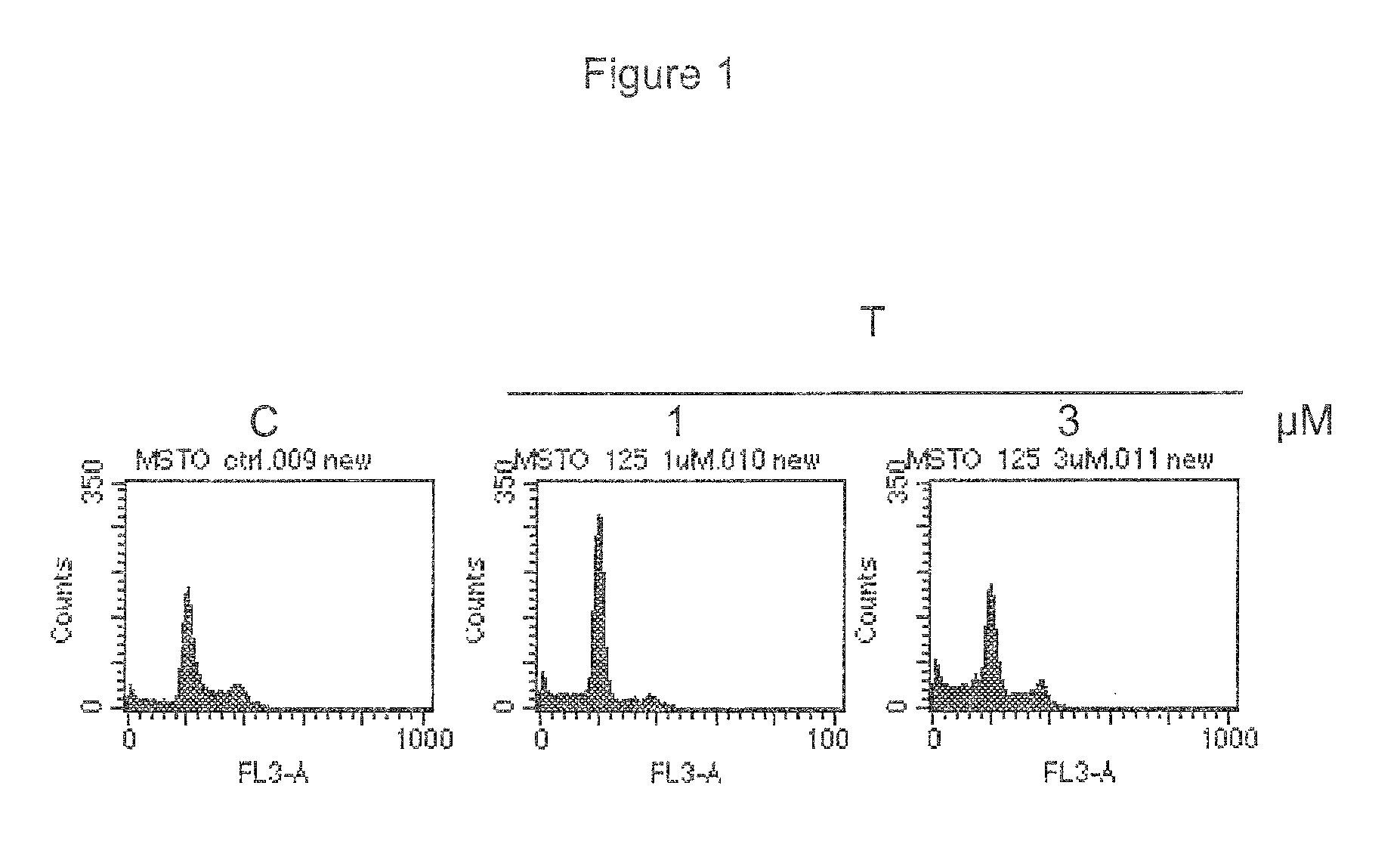
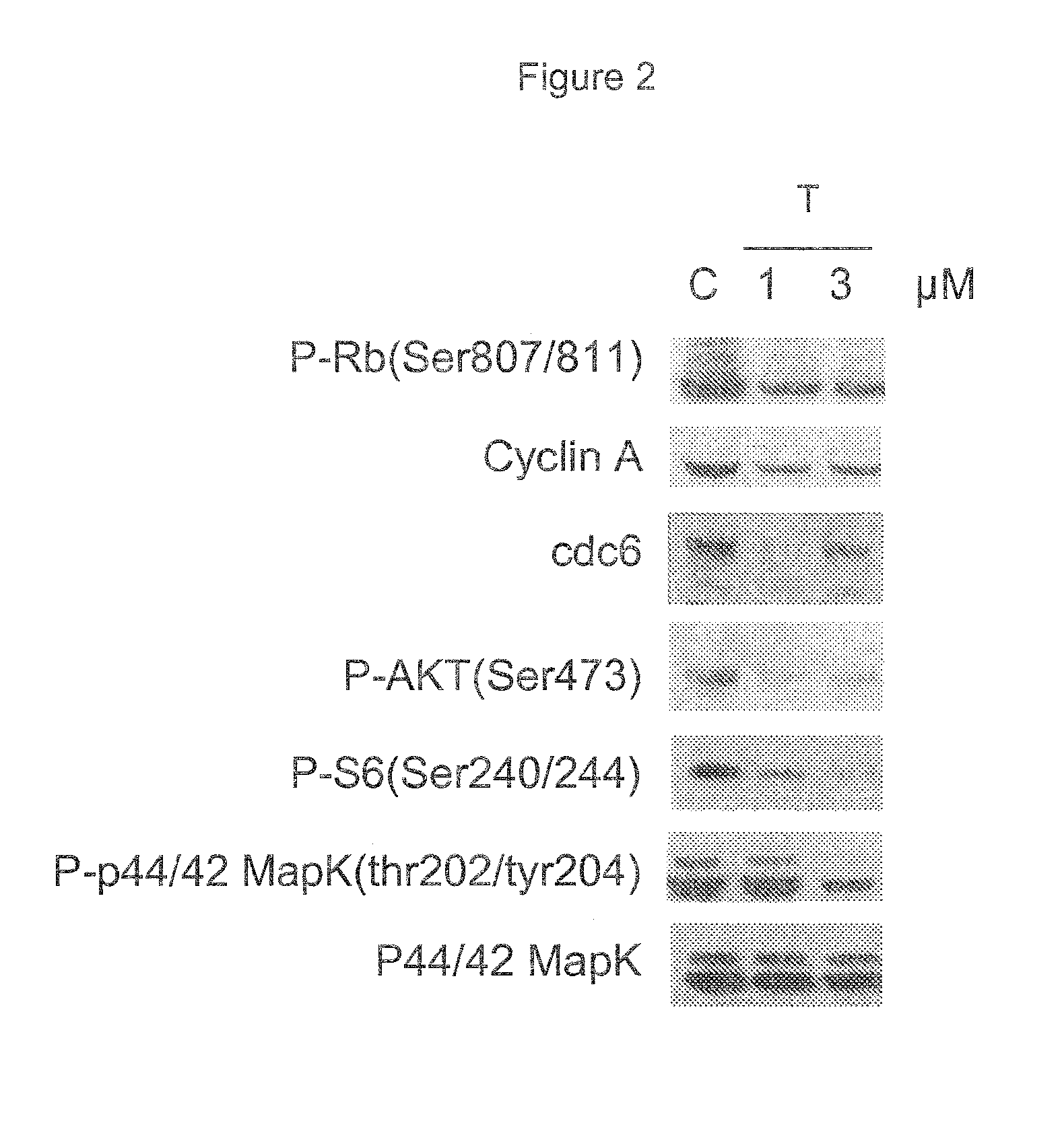
CDK inhibitor for the treatment of mesothelioma
ActiveUS8912194B2Prevent proliferationOrganic active ingredientsOrganic chemistryMesotheliomaCDK inhibitor
The invention provides a low molecular weight ATP-competitive CDK inhibitor for use in the treatment of mesothelioma. The compound can be administered together with one or more cytotoxic or cytostatic agents.
Owner:NERVIANO MEDICAL SERVICES SRL
Use of CDK inhibitor for the treatment of glioma
ActiveUS8946226B2Prevent proliferationReduce molecular weightOrganic active ingredientsOrganic chemistryGlioblastomaCytotoxicity
Owner:NERVIANO MEDICAL SERVICES SRL
Polyamine analog conjugates and quinone conjugates as therapies for cancers and prostate diseases
Peptide conjugates in which cytocidal and cytostatic agents, such as polyamine analogs or naphthoquinones, are conjugated to a polypeptide recognized and cleaved by enzymes such as prostate-specific antigen (PSA) and cathepsin B are provided, as well as compositions comprising these conjugates. Methods of using these conjugates in the treatment of prostate diseases are also provided.
Owner:CELLGATE
Novel compositions and methods for cancer treatment
ActiveUS20100310503A1Organic active ingredientsPeptide/protein ingredientsCancer treatmentCancer research
Owner:SUMITOMO DAINIPPON PHARMA ONCOLOGY INC
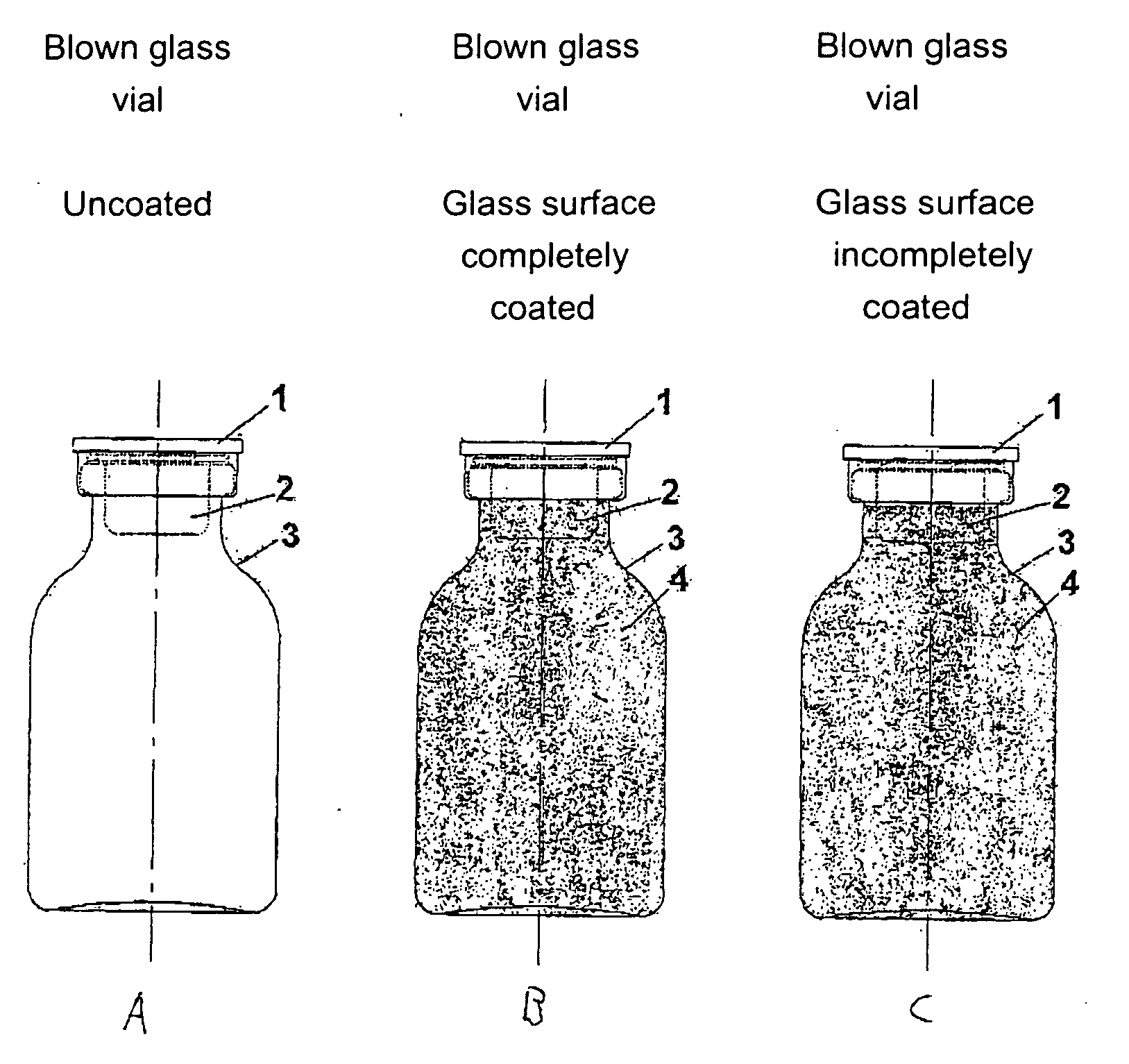
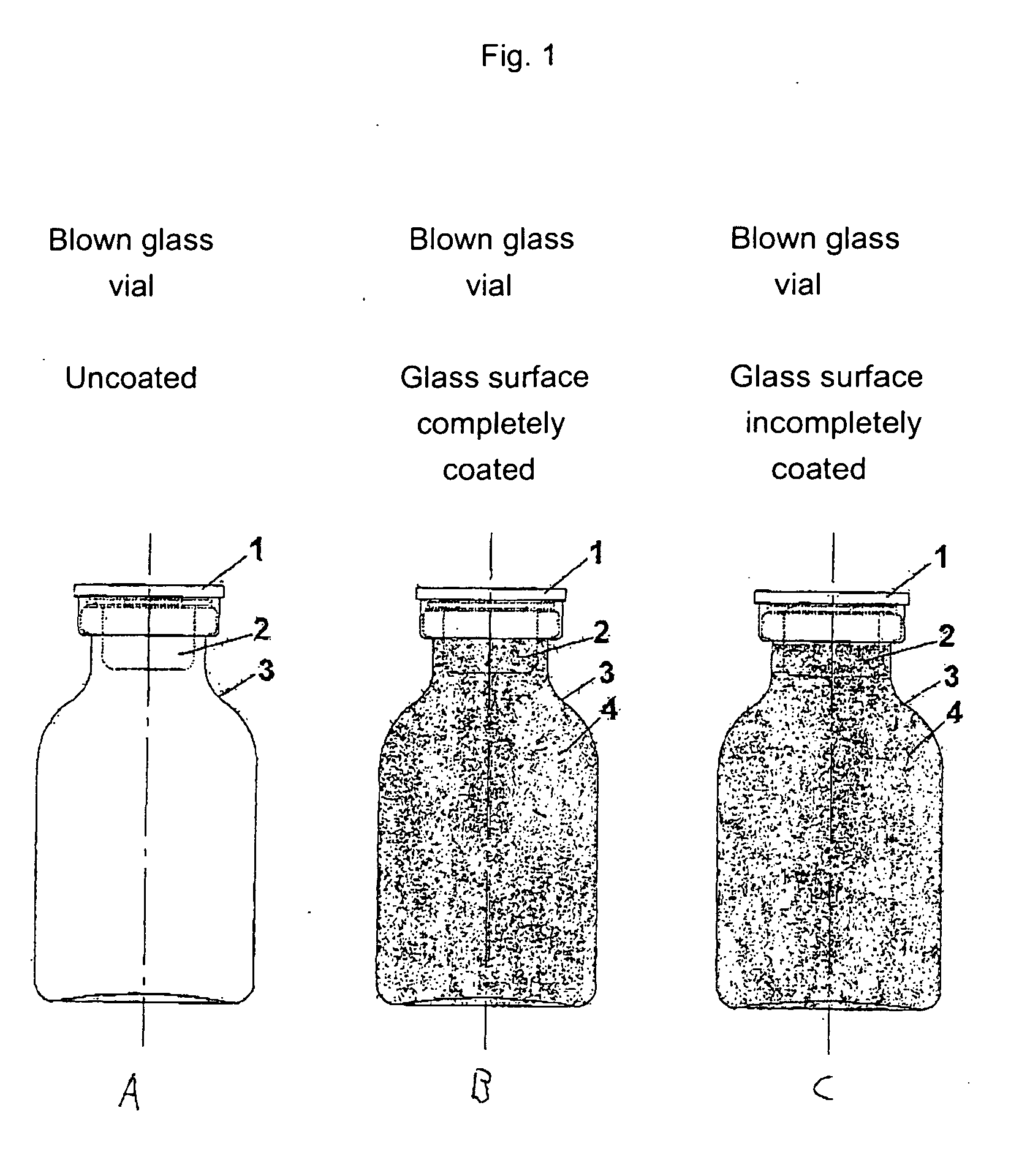
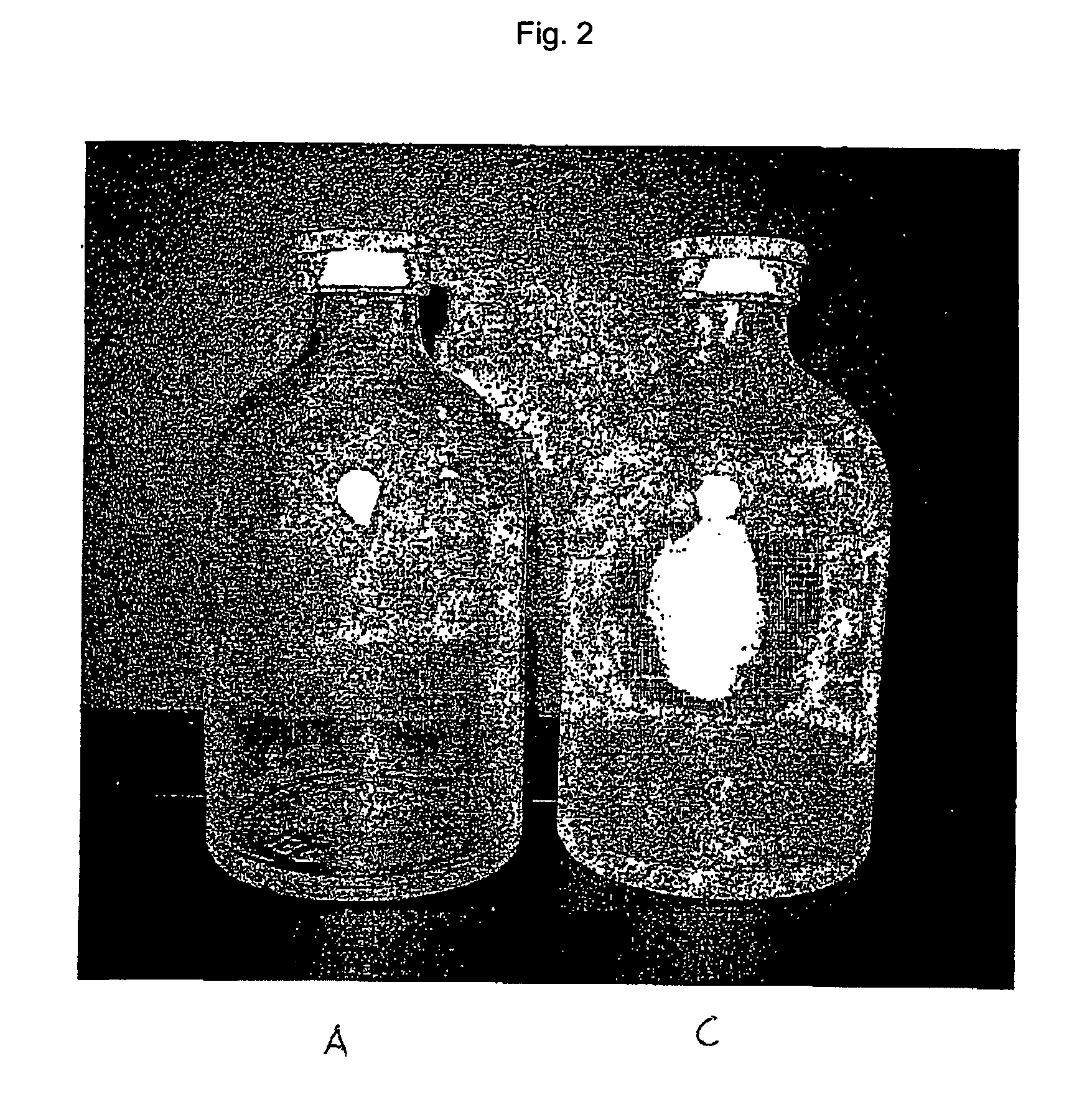
Safety containers for biologically active substances and method for producing said container
InactiveUS20070010700A1Increased and high fracture strengthSimple and rapid to carry-outClosure capsCoverings/external coatingsHigh fractureUltimate tensile strength
The invention relates to safety containers for biologically active substances, in particular cytostatic agents, said container having increased or higher fracture strength and shatterproof qualities, in addition to an uncontaminated exterior. The invention also relates to a method for producing said containers and to the use of a medium containing at least one polymer for decontaminating the exterior of a container that is filled with a biologically active substance, sealed and optionally labelled.
Owner:BAXTER INT INC +1
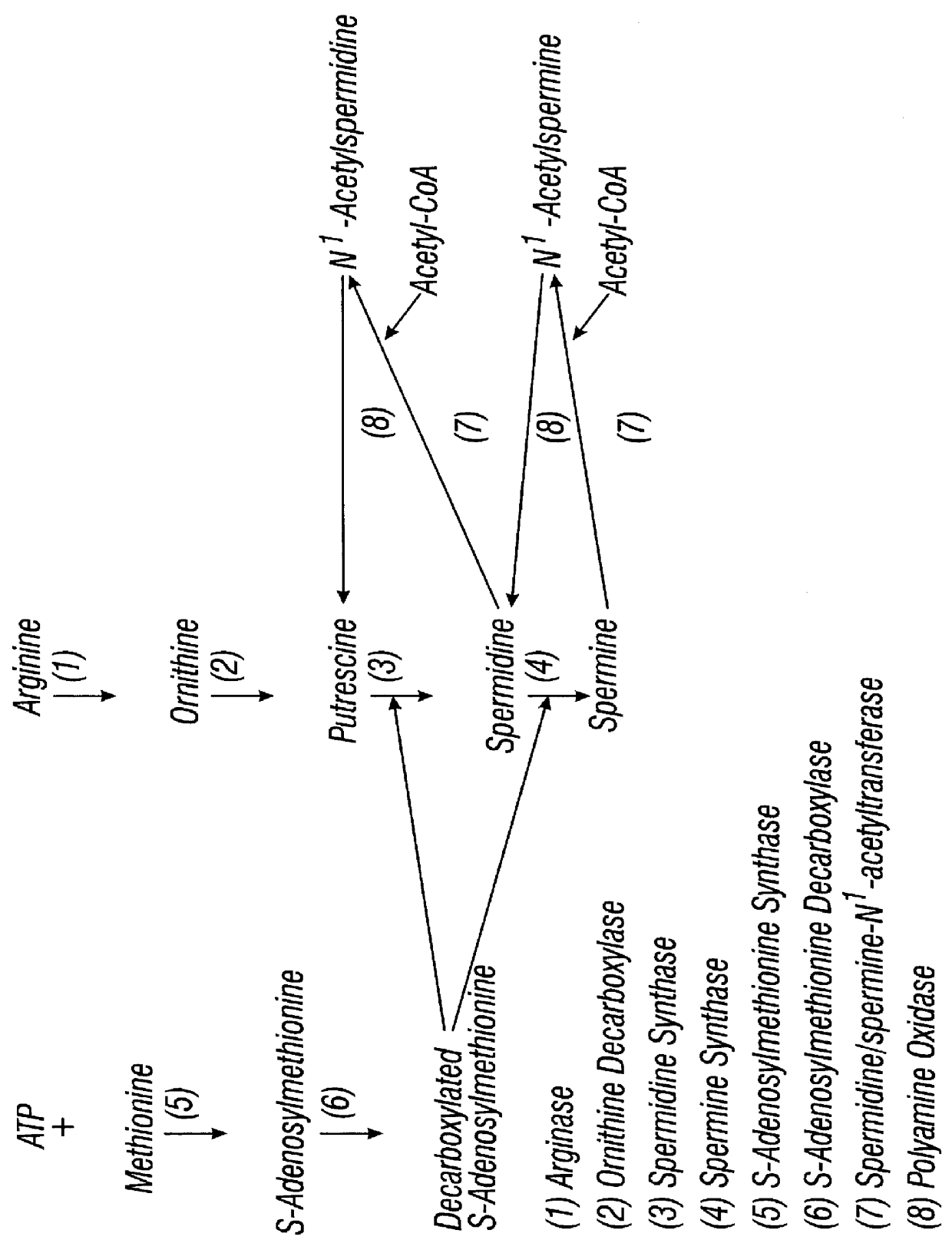
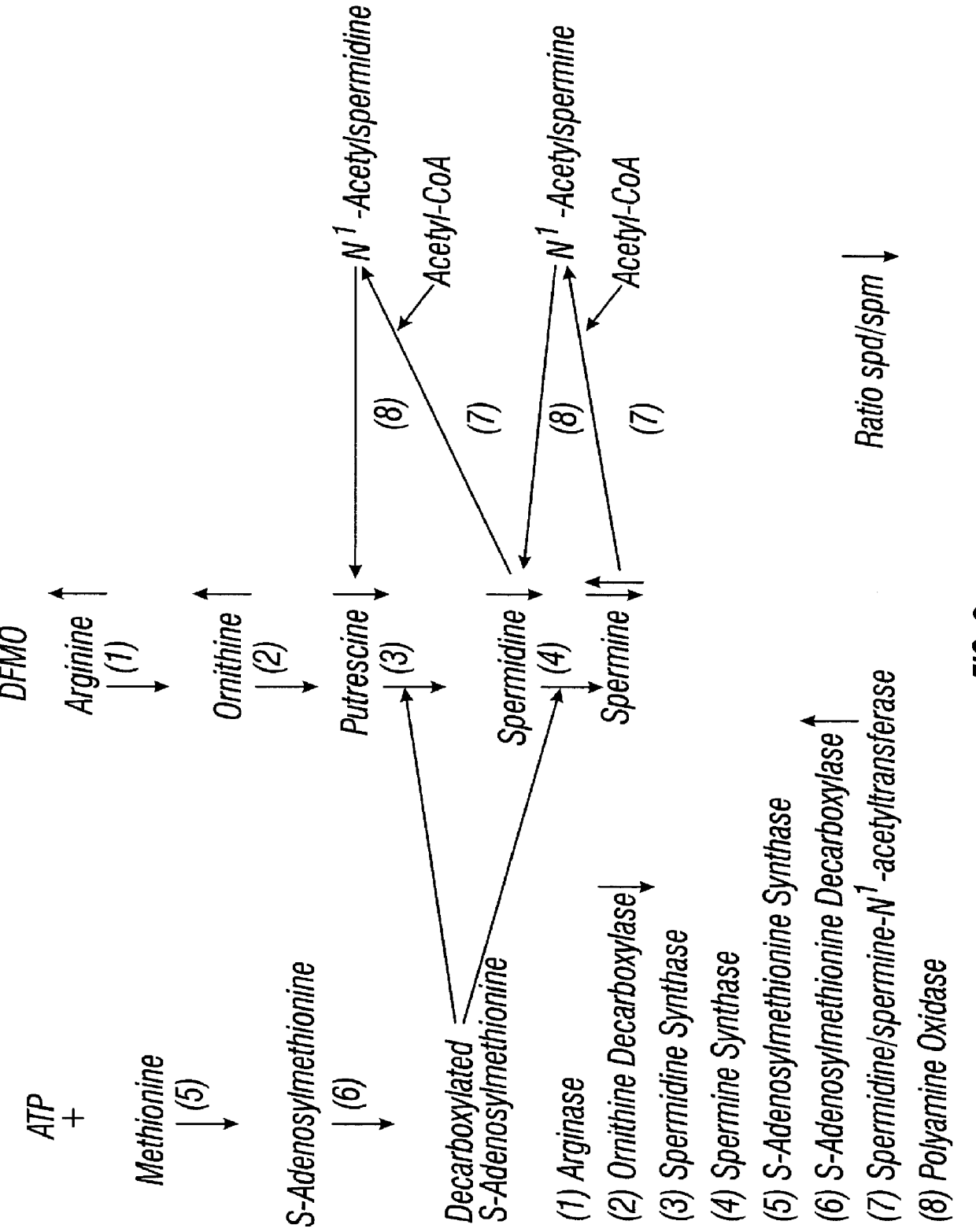
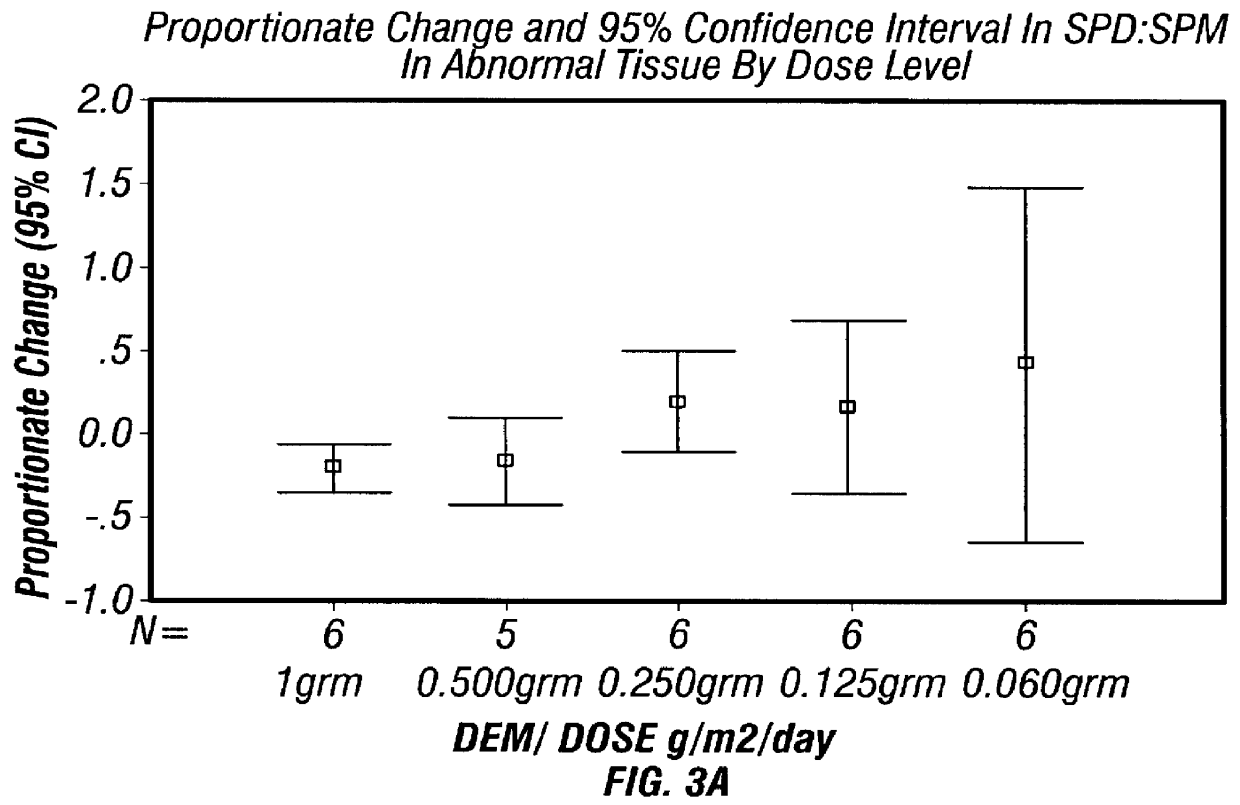
DFMO for the treatment or prevention of cervical intraepithelial neoplasia
InactiveUS6166079AReduce riskInhibit transformationBiocidePeptide/protein ingredientsCytotoxicityCervical intraepithelial neoplasia
Methods for treating, preventing, controlling the growth of and / or reducing the risk of developing cervical cancer, particularly in patients with cervical intraepithelial neoplasia are provided employing pharmaceutically acceptable preparations of DFMO. Methods for treating a patient having cervical intraepithelial neoplasia, which methods comprise administering DFMO alone or in combination with a cytotoxic or cytostatic agent, are also provided.
Owner:TEXAS SYST UNIV OF BOARD OF REGENTS THE +1
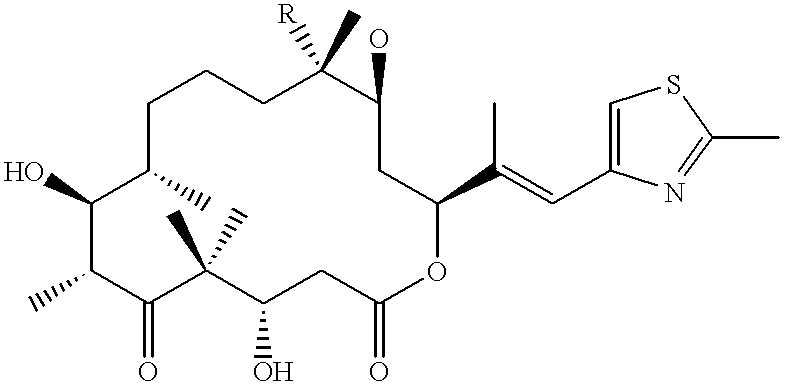
Fermentative preparation process for and crystal forms of cytostatics
The invention relates to a new process for concentrating epothilones in culture media, a new process for the production of epothilones, a new process for separating epothilones A and B and a new strain obtained by mutagenesis for the production of epothilones, as well as aspects related thereto. New crystal forms of epothilone B are also described.
Owner:NOVARTIS AG
Conjoint therapy for treating fibrotic diseases
ActiveUS20100111898A1Inhibition of activationReduce inhibitionAntibacterial agentsOrganic active ingredientsDiseaseCombined Modality Therapy
The present invention relates to improved methods of treating fibrotic or fibroproliferative disorders. Conjoint therapies are provided comprising the combination of one or more fibrocyte suppressors and one or more profibrotic factor antagonists or anti-fibrotic agents.
Owner:PROMEDIOR
FAP-activated anti-tumor compounds
InactiveUS20020155565A1Sugar derivativesPeptide/protein ingredientsAbnormal tissue growthCytotoxicity
The invention relates to a prodrug that is capable of being converted into a drug by the catalytic action of human fibroblast activation protein (FAPalpha), said prodrug having a cleavage site which is recognized by FAPalpha, and said drug being cytotoxic or cytostatic under physiological conditions, wherein said prodrug comprises an oligomeric part comprising at least two amino carboxylic residues, and a cytotoxic or cytostatic part, wherein the C-terminal amino carboxylic residue of the oligomeric part is an acyclic amino acid, the nitrogen atom of the amino function thereof is attached to a substituent being different from a hydrogen atom, and the C-terminal carboxy function thereof is linked to the cytotoxic or cytostatic part by an amide bond.
Owner:BOEHRINGER INGELHEIM PHARM KG
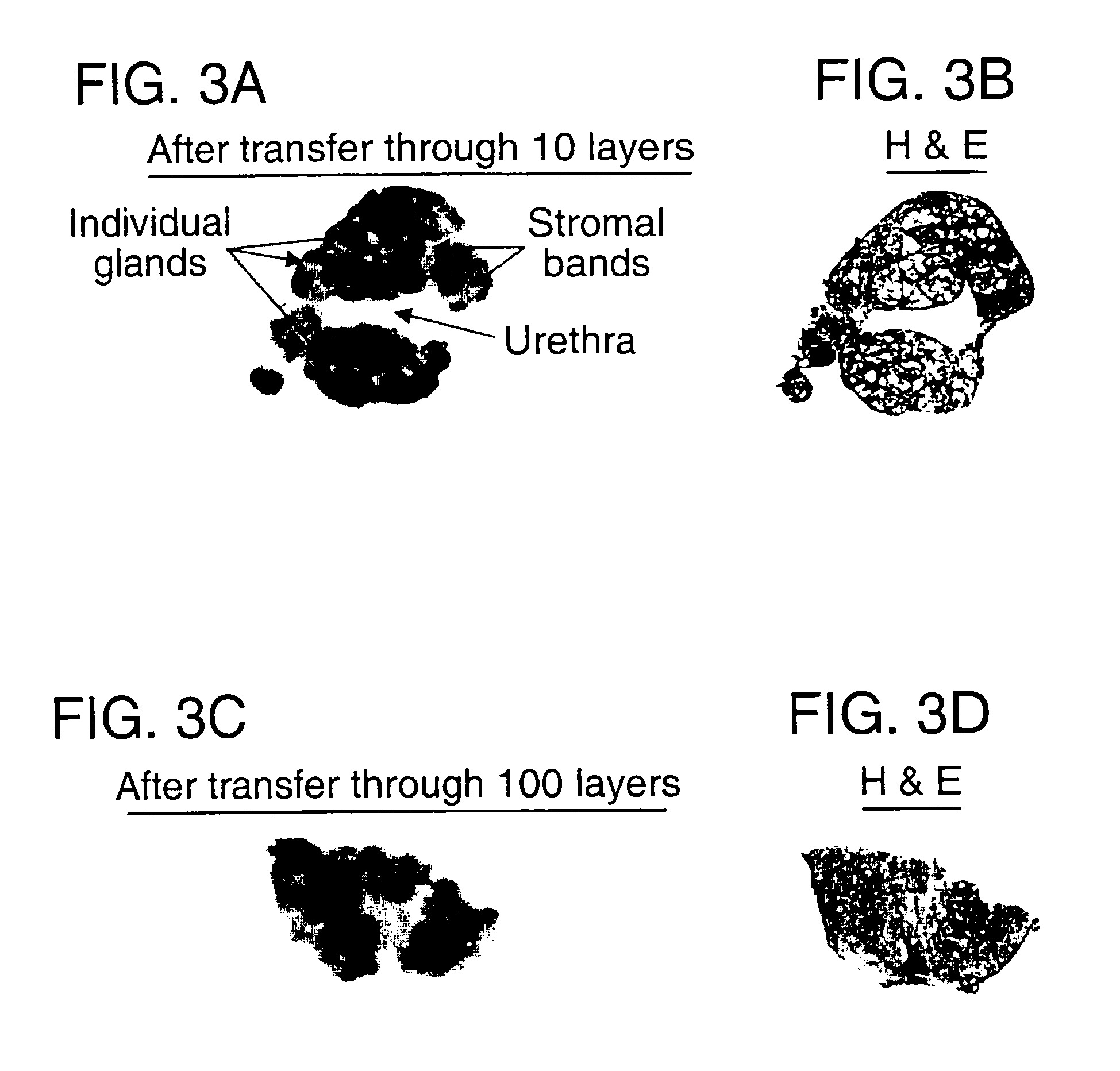
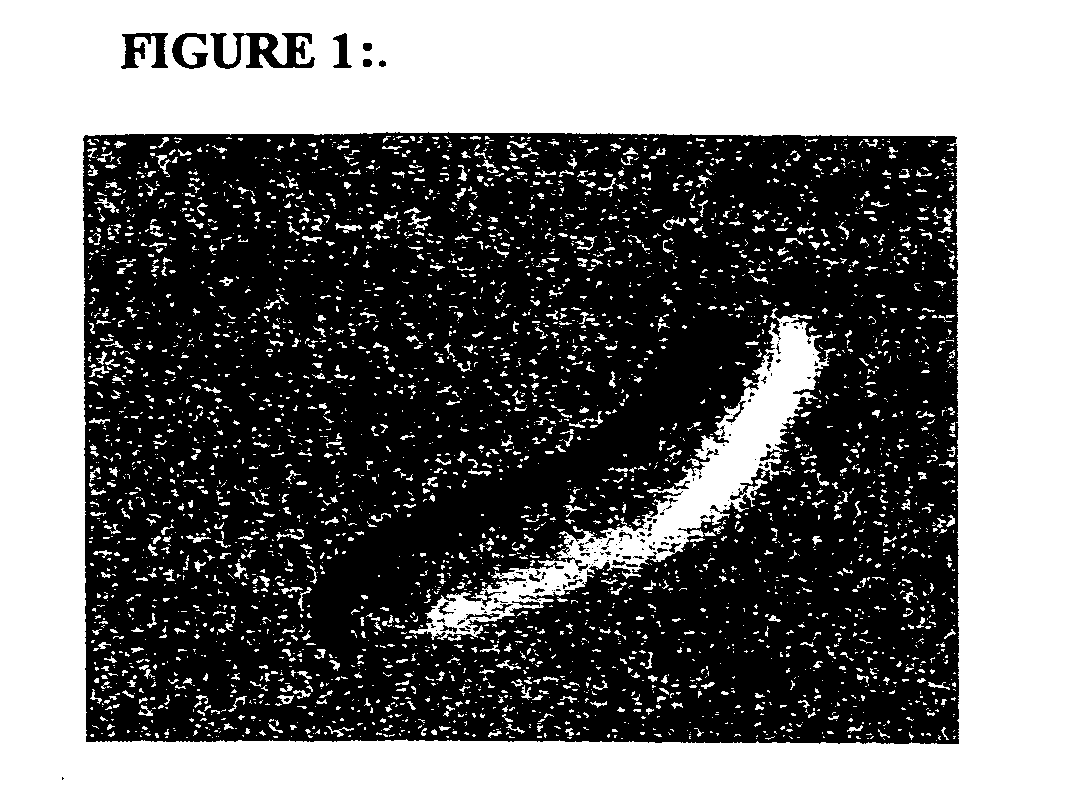
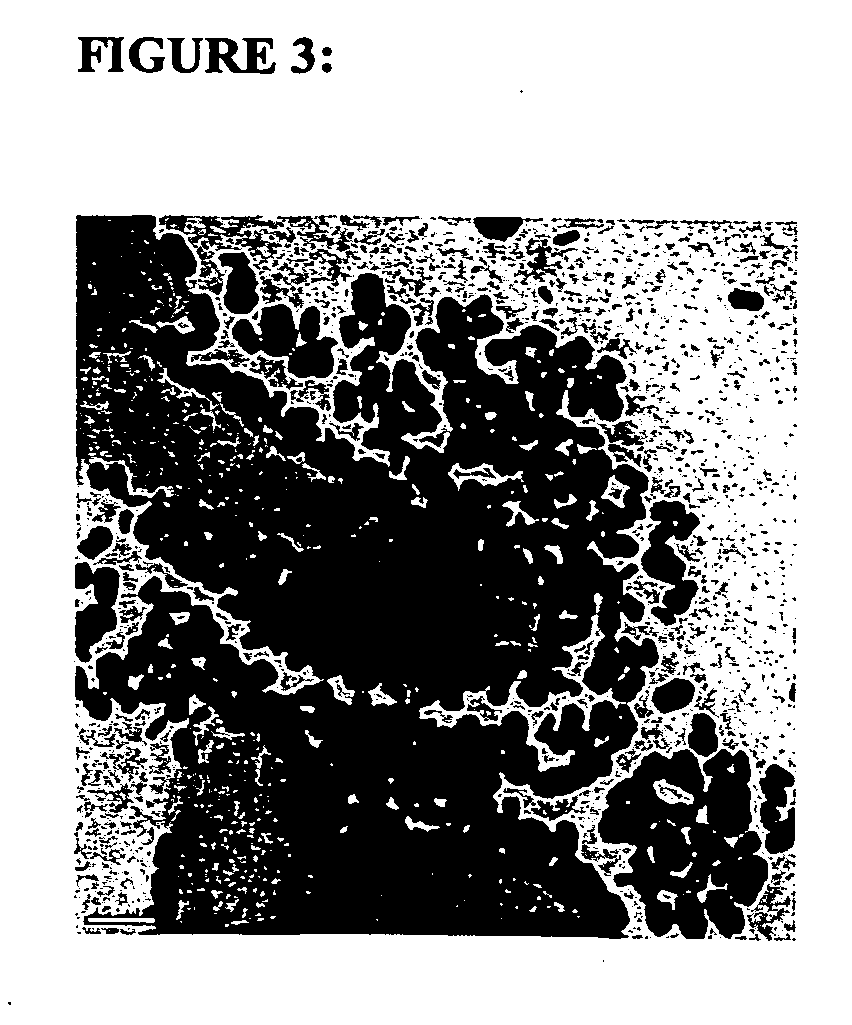
Layered device with capture regions for cellular analysis
InactiveUS7214477B1Easy to detectMicrobiological testing/measurementBiological testingCellular architectureElectrophoresis
The present invention involves methods, systems, and devices for analyzing a biological material, such as a cellular or other specimen. The method includes placing the specimen on a substrate having different capture regions, such as contiguous layers, wherein the different capture regions of the substrate contain different identification molecules, and transferring components of the specimen through the capture regions under conditions that allow the components to interact with different identification molecules in the different regions of the substrate. The components of the specimen can be transferred through the different layers (or other regions) of the substrate by capillary action of a solution moving through the cellular specimen or by electrophoresis. The transfer of components of the specimen through the substrate may occur while maintaining a geometric correspondence to the specimen, such as the cytoarchitecture of a cellular specimen, for example by moving the components through parallel layers having positions that correspond to positions within the specimen. When the cellular architecture of the specimen is maintained, a correlation between the different identification molecules and the components of the cellular specimens may be made. The analysis can occur with one or more different discrete (for example cellular) specimens on a surface of the substrate. Examples of cellular specimens include, but are not limited to tissue sections, particularly tumor tissue sections. The cellular specimen can also include cultured cells or a cytology sample. Cytostat tissue sections cut slightly thicker than usual, that is about 25 to about 50 μm, improves the ability to detect molecules of moderate and low level abundance.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Methods and compositions for the treatment of diseases characterized by pathological calcification
InactiveUS20060069068A1Good curative effectBiocidePeptide/protein ingredientsGrowth retardantCalcification
Methods and compositions are provided which contains preparations of calcium chelators, bisphosphonates, antibiotics, antimicrobial agents, cytostatic agents, calcium ATPase and pyrophosphatase pump inhibitors, calcium phosphate-crystal dissolving agents, agents effective against calcium phosphate-crystal nucleation and crystal growth, and / or a combination of supportive agents and which may be used for treating and or reducing pathological calcifications, the growth of Nanobacterium and calcification-induced diseases including, but not limited to, Arteriosclerosis, Atherosclerosis, Coronary Heart Disease, Chronic Heart Failure, Valve Calcifications, Arterial Aneurysms, Calcific Aortic Stenosis, Transient Cerebral Ischemia, Stroke, Peripheral Vascular Disease, Vascular Thrombosis, Dental Plaque, Gum Disease (dental pulp stones), Salivary Gland Stones, Chronic Infection Syndromes such as Chronic Fatigue Syndrome, Kidney and Bladder Stones, Gall Stones, Pancreas and Bowel Diseases (such as Pancreatic Duct Stones, Crohn's Disease, Colitis Ulcerosa), Liver Diseases (such as Liver Cirrhosis, Liver Cysts), Testicular Microliths, Chronic Calculous Prostatitis, Prostate Calcification, Calcification in Hemodialysis Patients, Malacoplakia, Autoimmune Diseases. Erythematosus, Scleroderma, Dermatomyositis, Antiphospholipid Syndrome, Arteritis Nodosa, Thrombocytopenia, Hemolytic Anemia, Myelitis, Livedo Reticularis, Chorea, Migraine, Juvenile Dermatomyositis, Grave's Disease, Hypothyreoidism, Type 1 Diabetes Mellitus, Addison's Disease, Hypopituitarism, Placental and Fetal Disorders, Polycystic Kidney Disease, Glomerulopathies, Eye Diseases (such as Corneal Calcifications, Cataracts, Macular Degeneration and Retinal Vasculature-derived Processes and other Retinal Degenerations, Retinal Nerve Degeneration, Retinitis, and Iritis), Ear Diseases (such as Otosclerosis, Degeneration of Otoliths and Symptoms from the Vestibular Organ and Inner Ear (Vertigo and Tinnitus)), Thyroglossal Cysts, Thyroid Cysts, Ovarian Cysts, Cancer (such as Meningiomas, Breast Cancer, Prostate Cancer, Thyroid Cancer, Serous Ovarian Adenocarcinoma), Skin Diseases (such as Calcinosis Cutis, Calciphylaxis, Psoriasis, Eczema, Lichen Ruber Planus), Rheumatoid Arthritis, Calcific Tenditis, Osteoarthritis, Fibromyalgia, Bone Spurs, Diffuse Interstitial Skeletal Hyperostosis, Intracranial Calcifications (such as Degenerative Disease Processes and Dementia), Erythrocyte-Related Diseases involving Anemia, Intraerythrocytic Nanobacterial Infection and Splenic Calcifications, Chronic Obstructive Pulmonary Disease, Broncholiths, Bronchial Stones, Neuropathy, Calcification and Encrustations of Implants, Mixed Calcified Biofilms, and Myelodegenerative Disorders (such as Multiple Sclerosis, Lou Gehrig's and Alzheimer's Disease) in humans and animals. The method comprises administering the various classes of compositions of the present invention, which together effectively inhibit or treat the development of calcifications in vivo.
Owner:CIFTCIOGLU NEVA
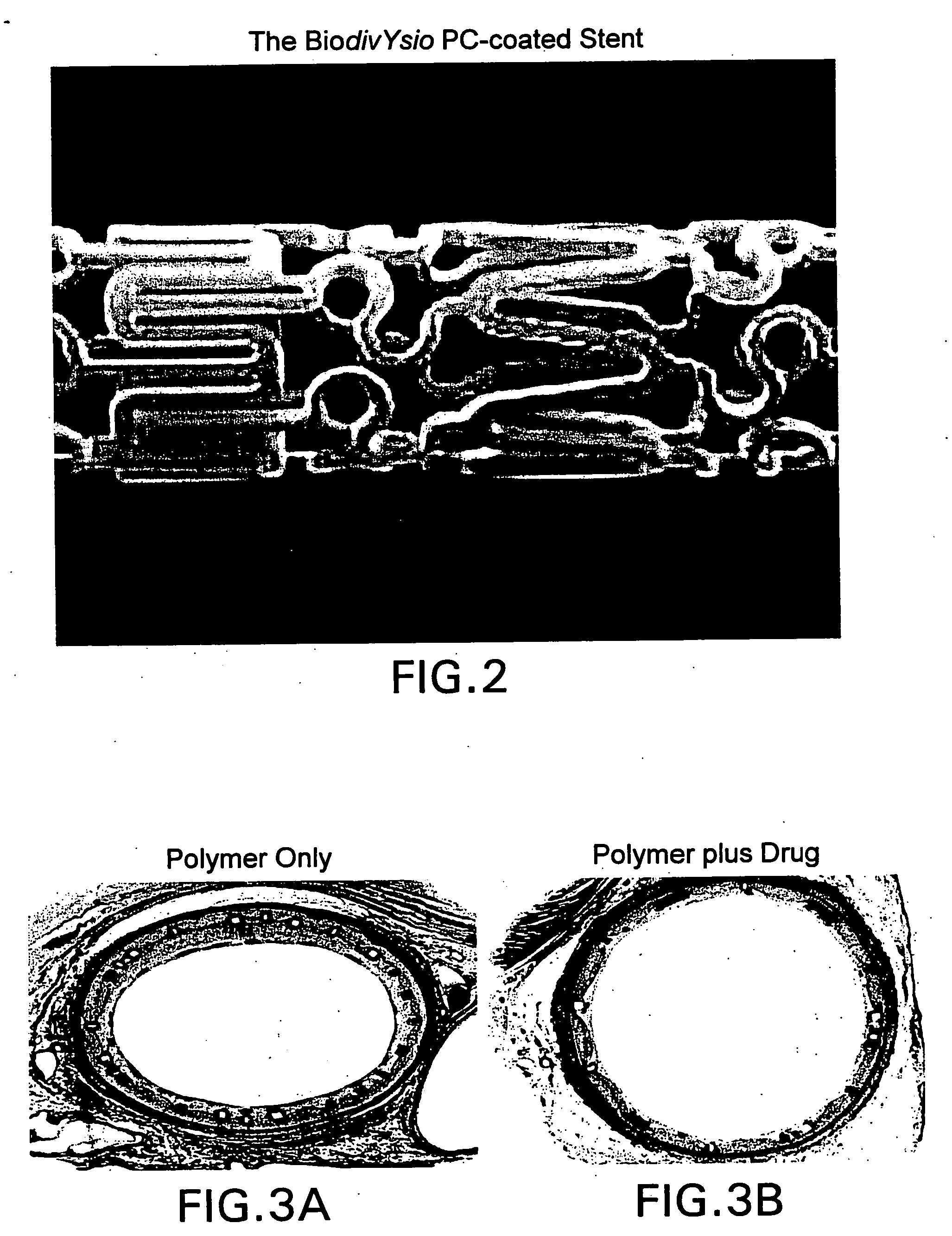
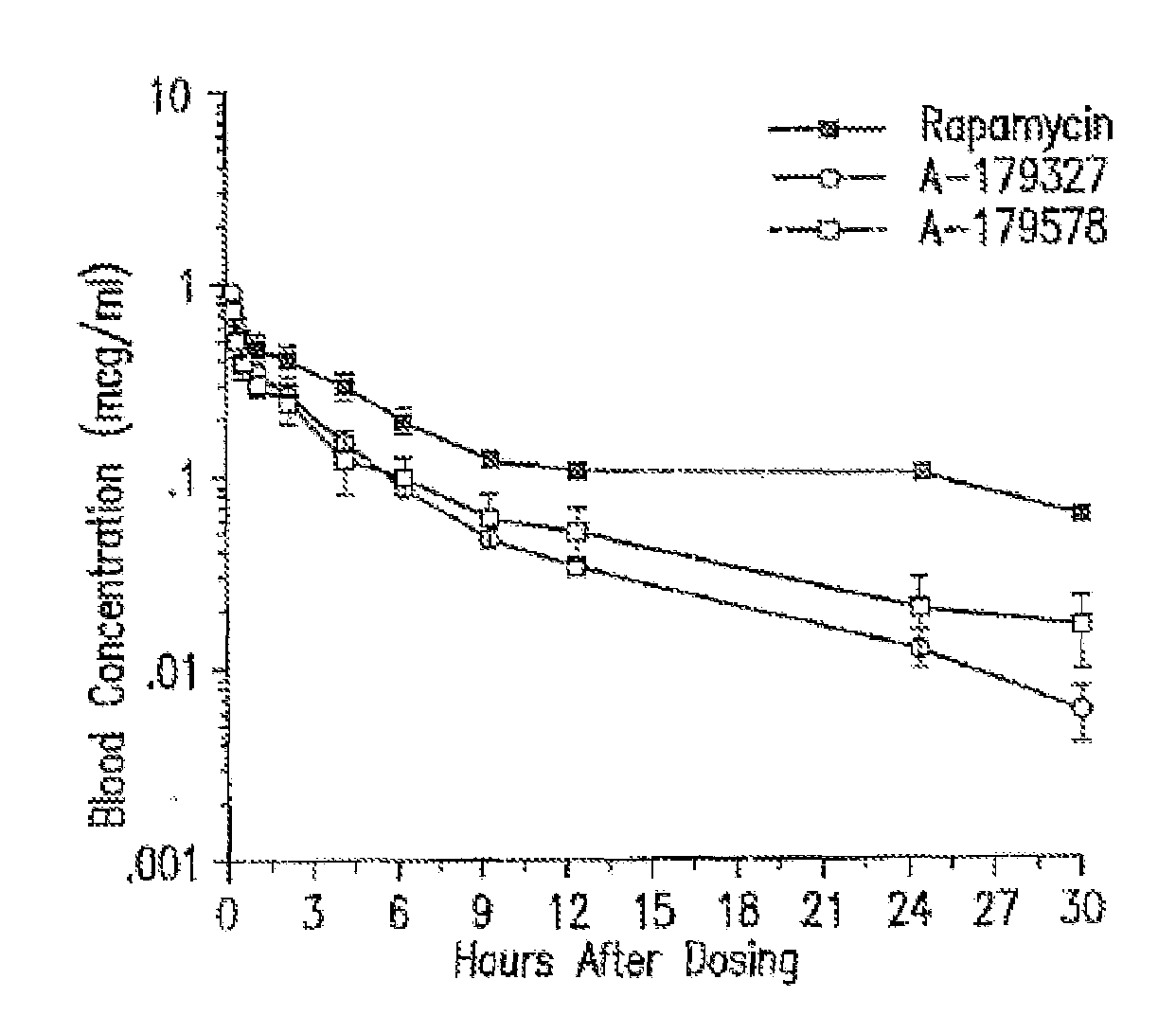
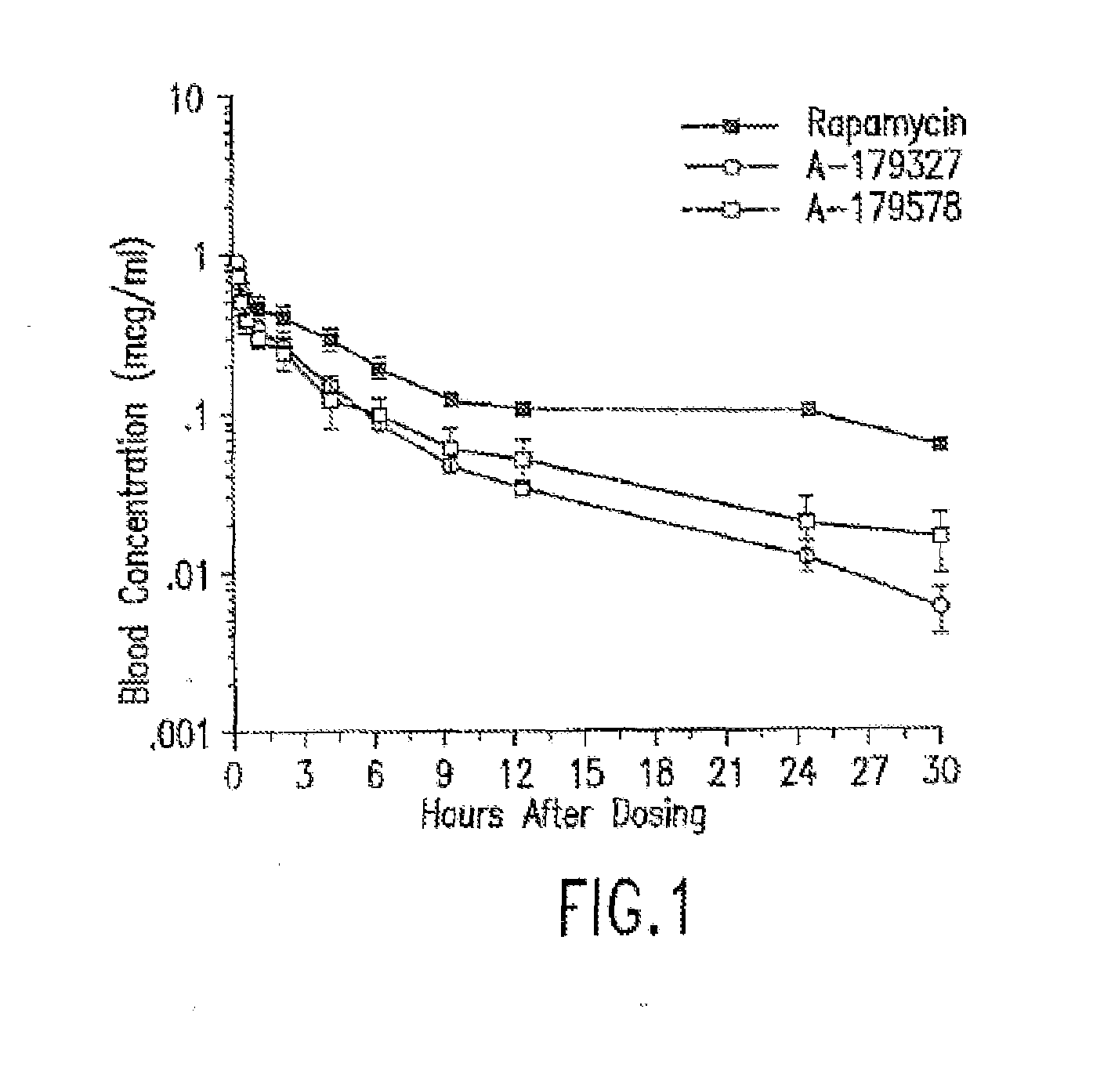
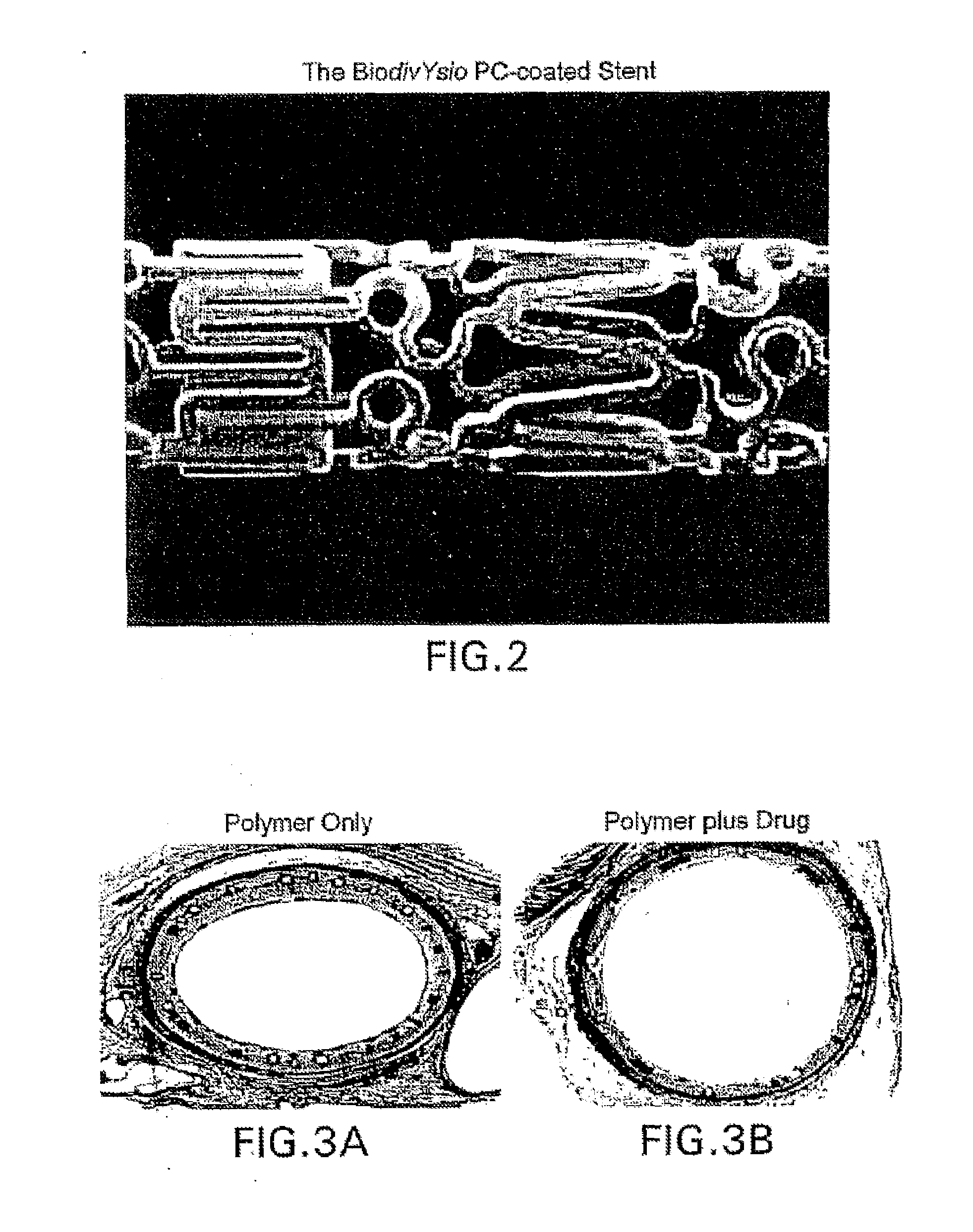
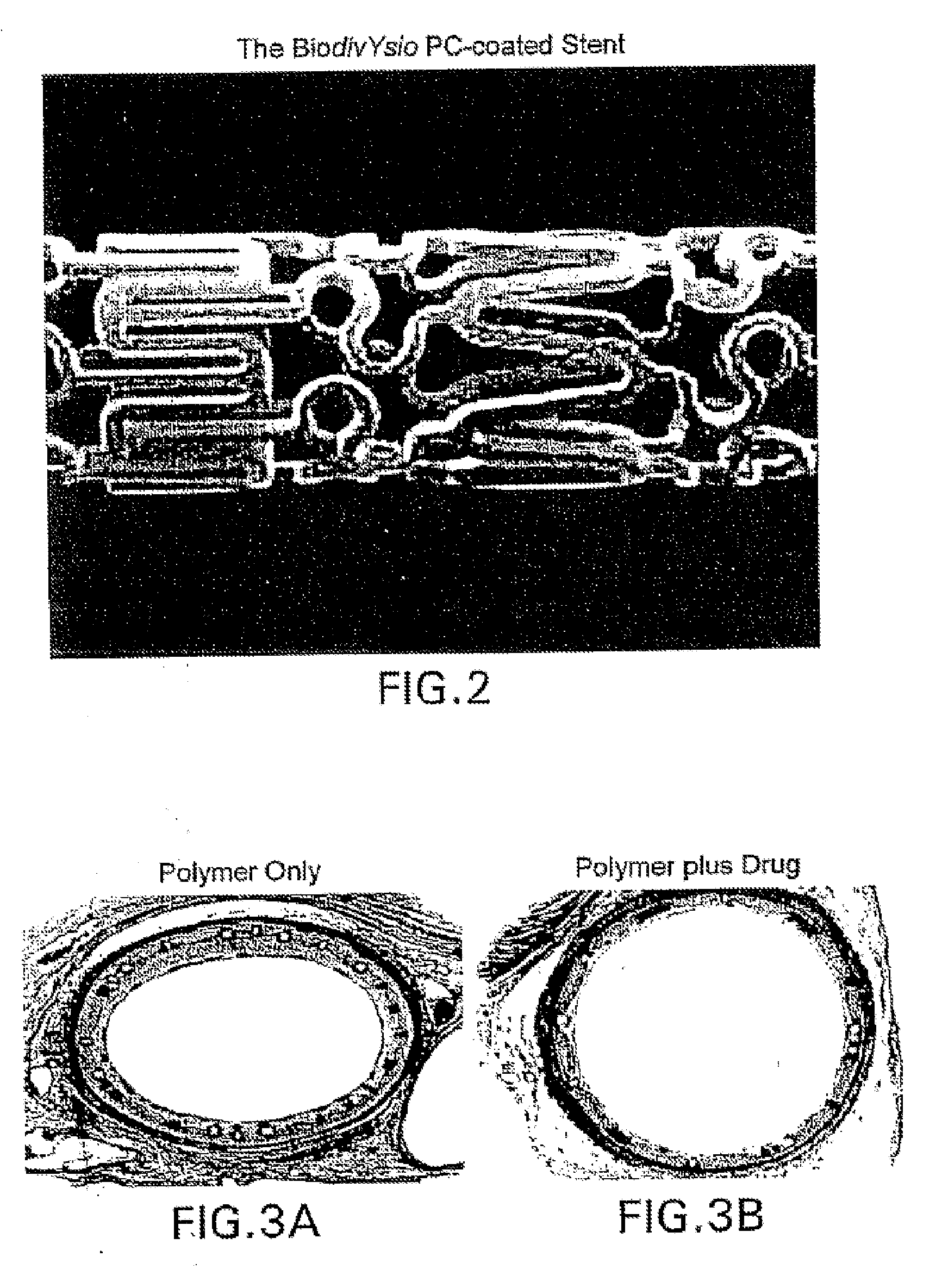
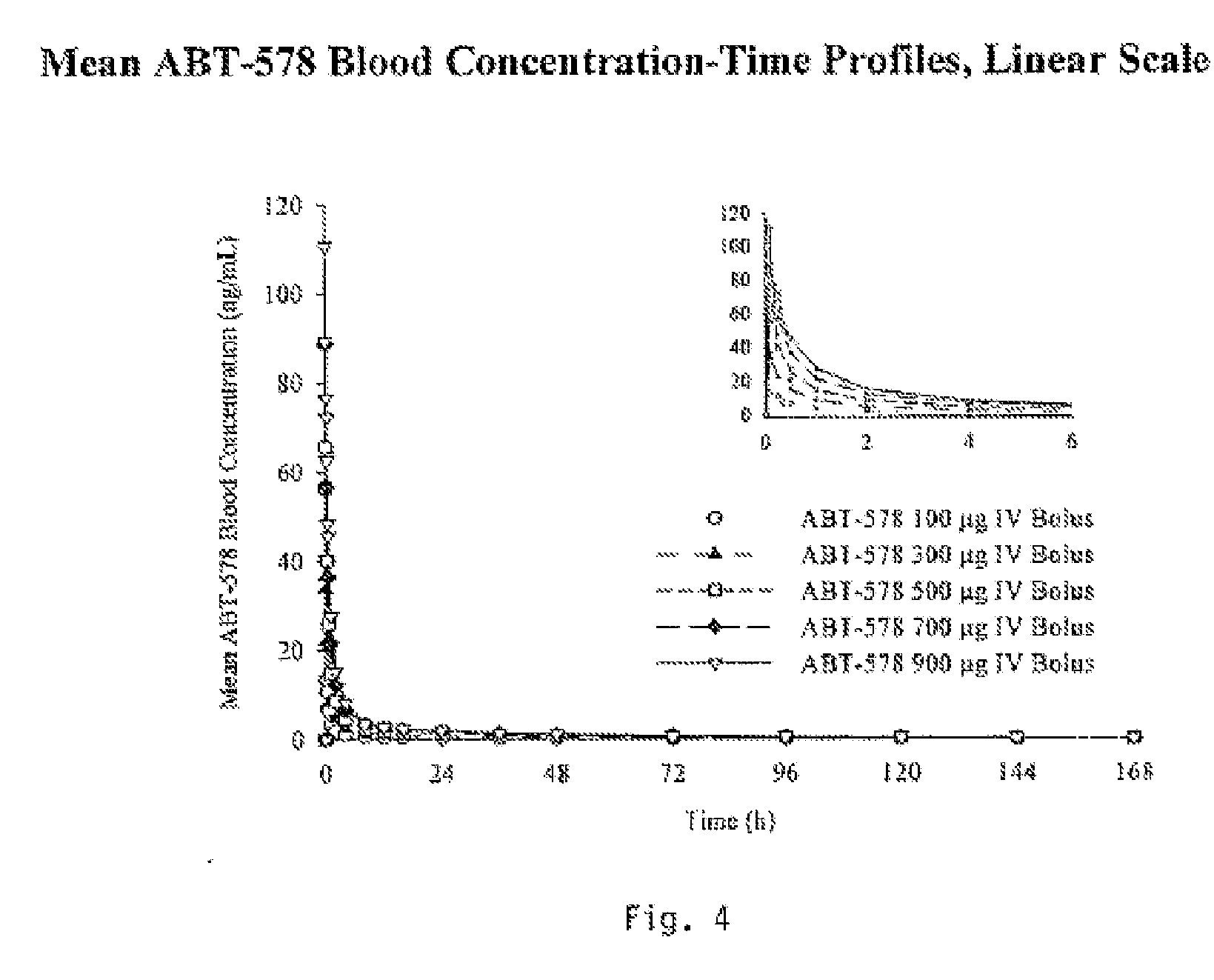
Compositions, systems, kits, and methods of administering rapamycin analogs with paclitaxel using medical devices
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
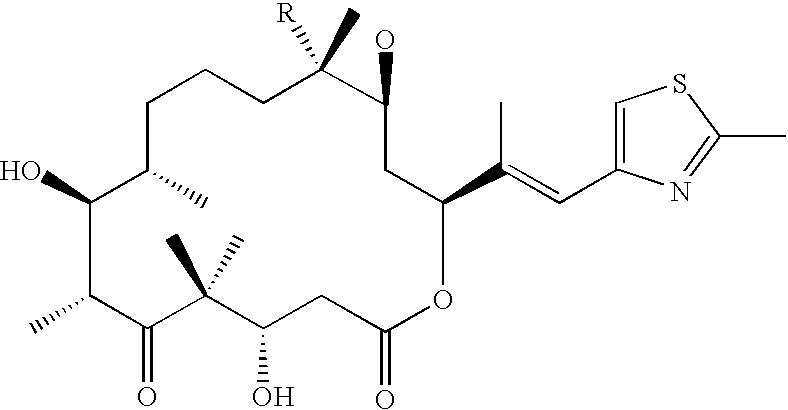
Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy
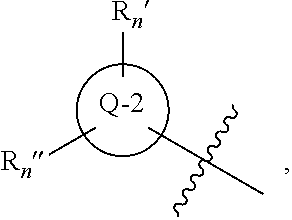
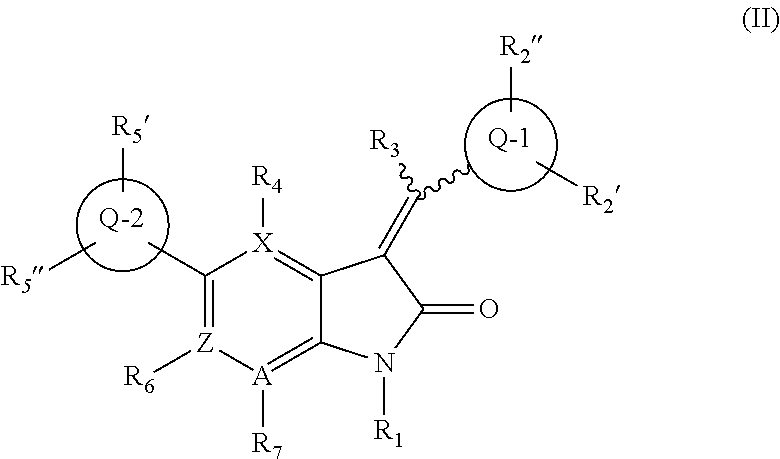
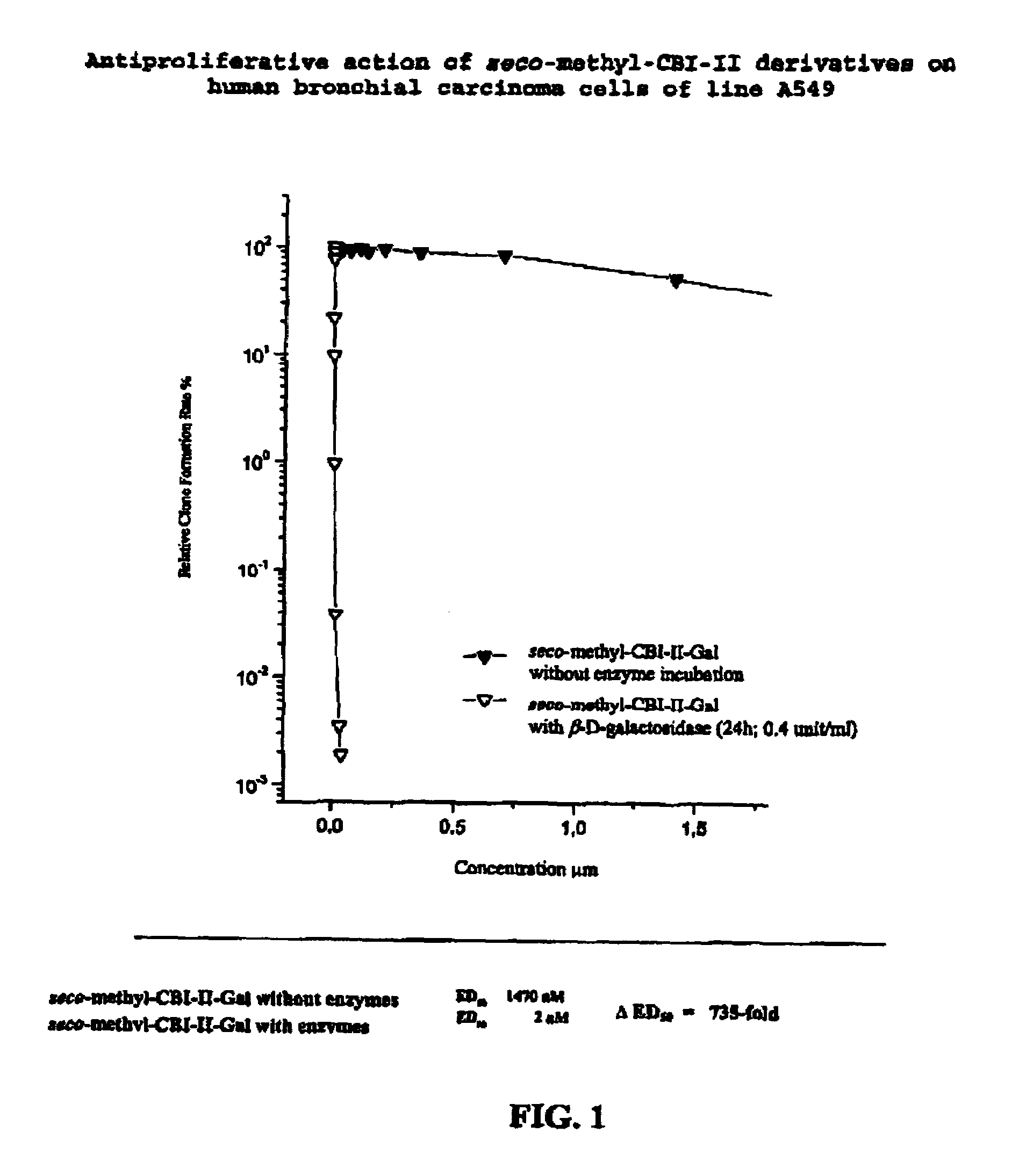
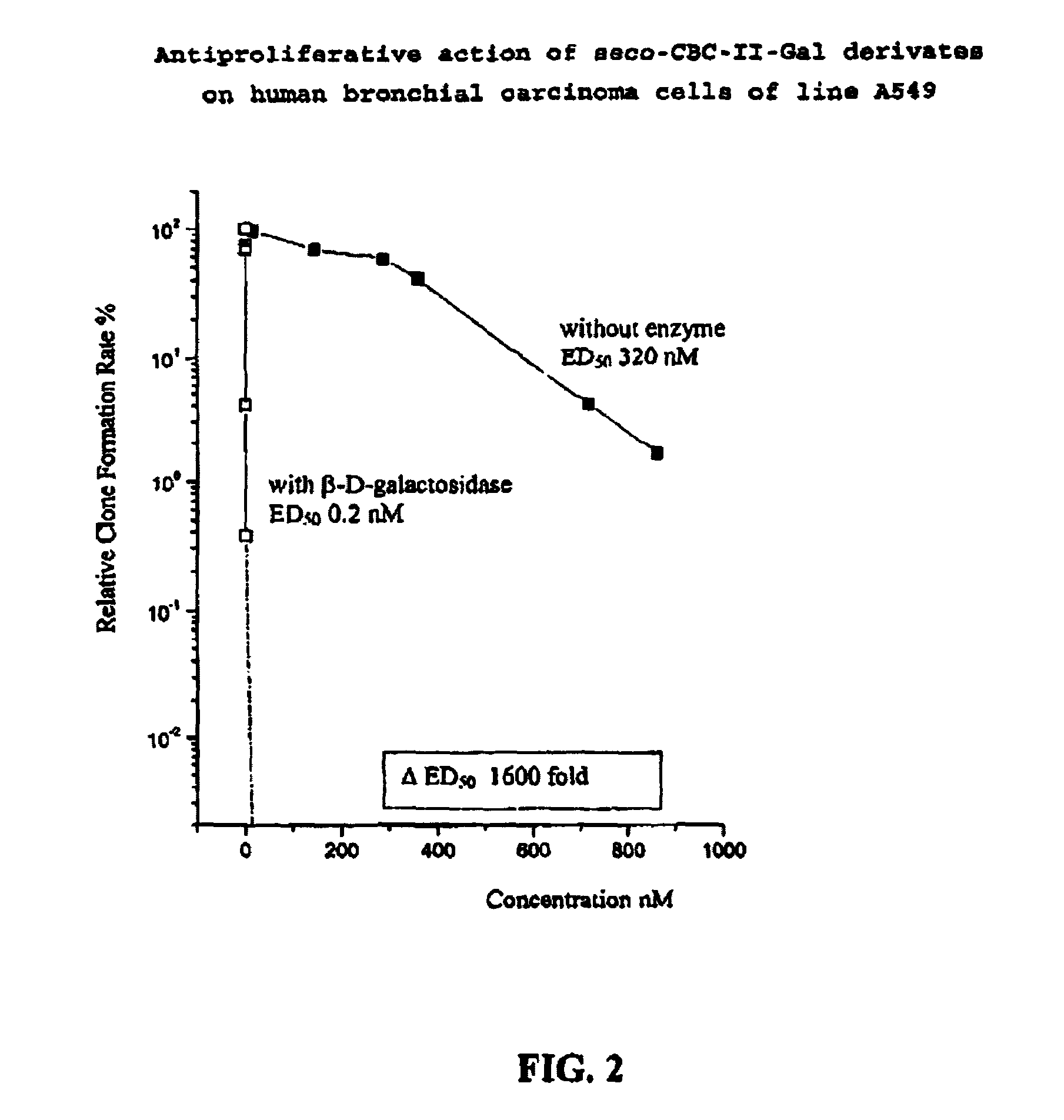
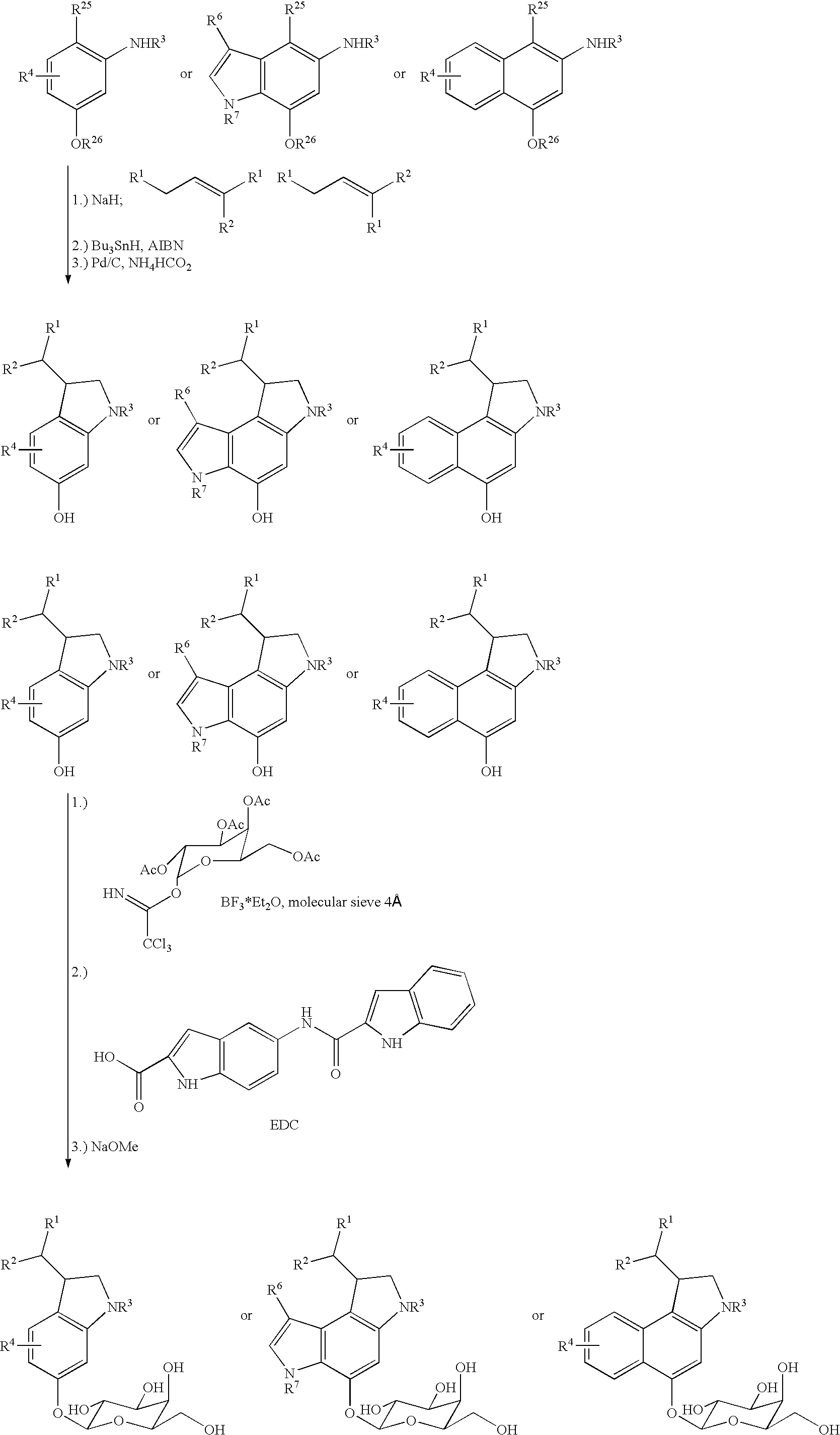
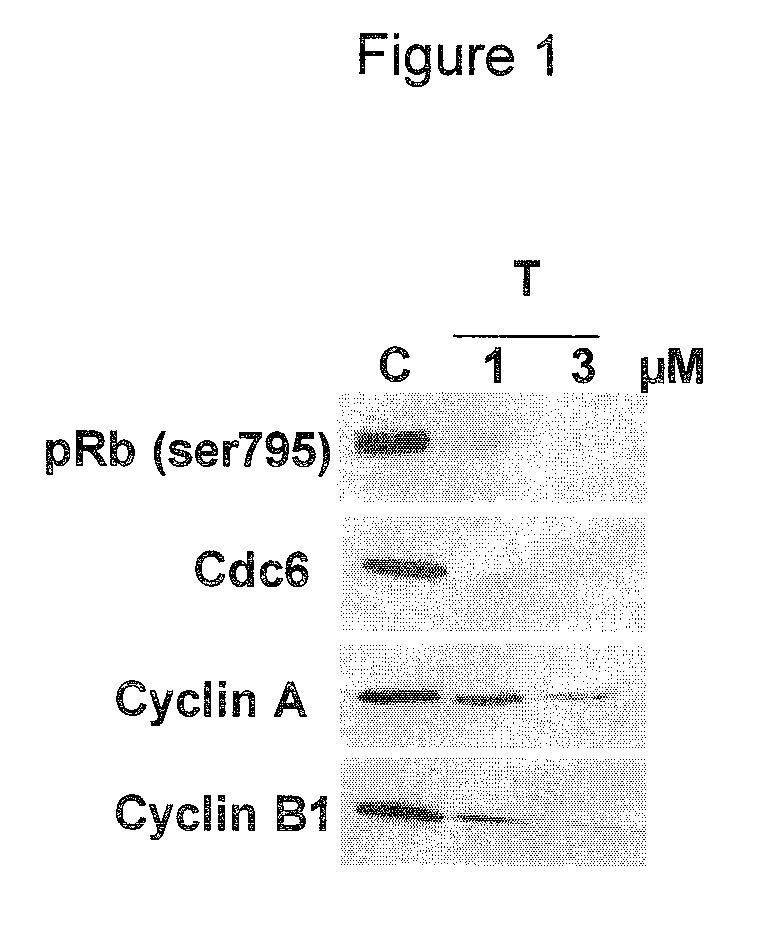
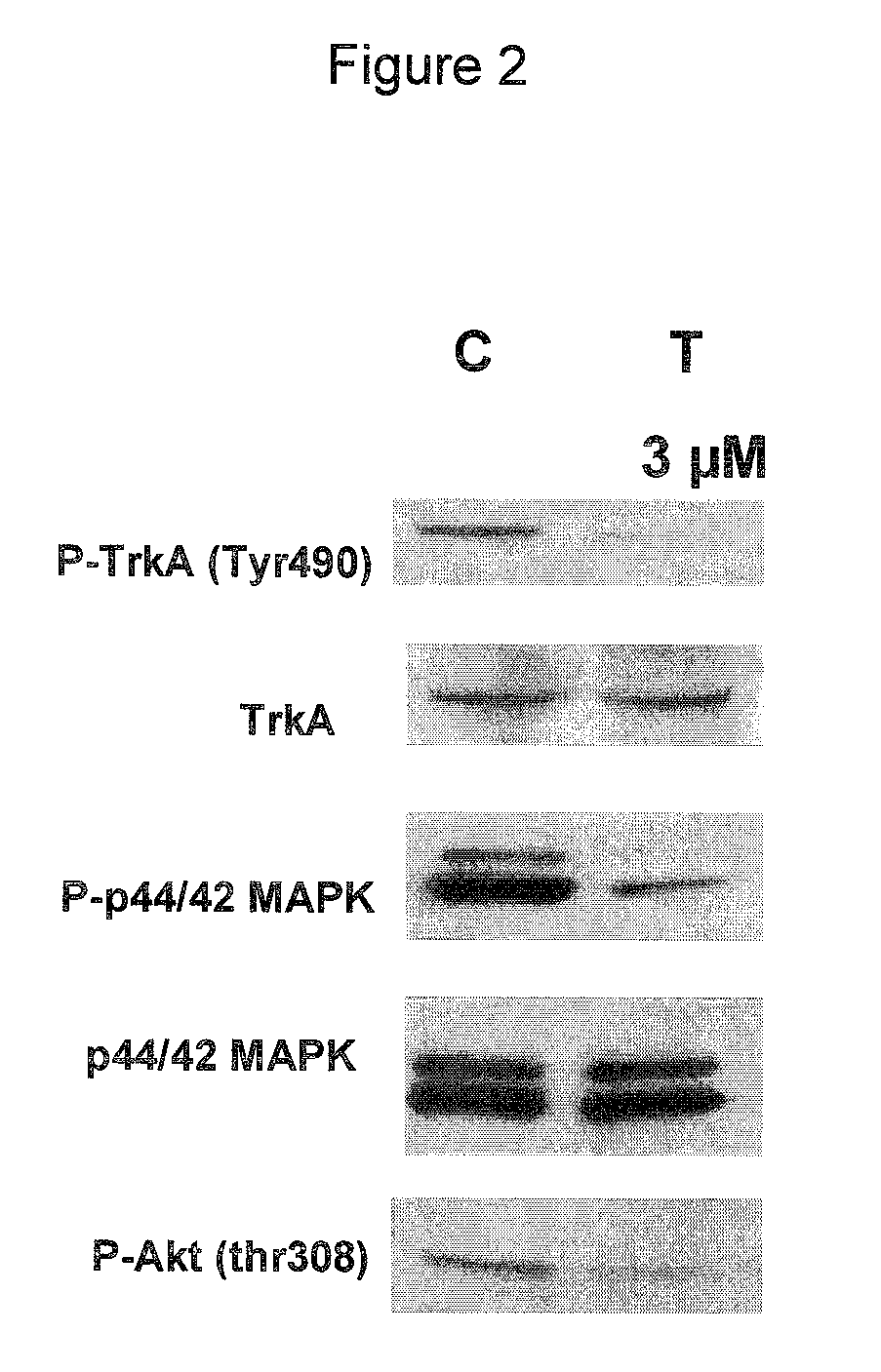
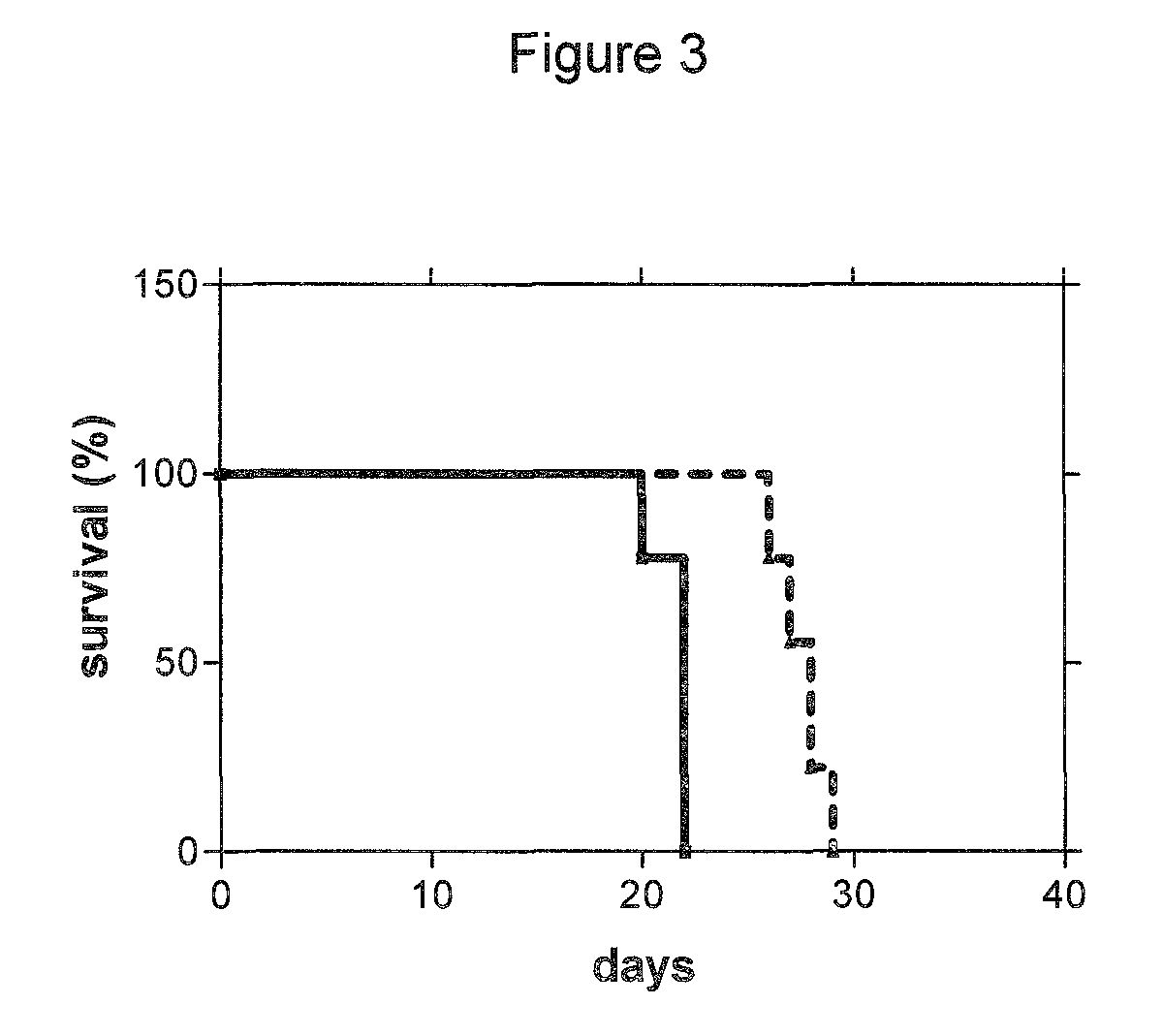
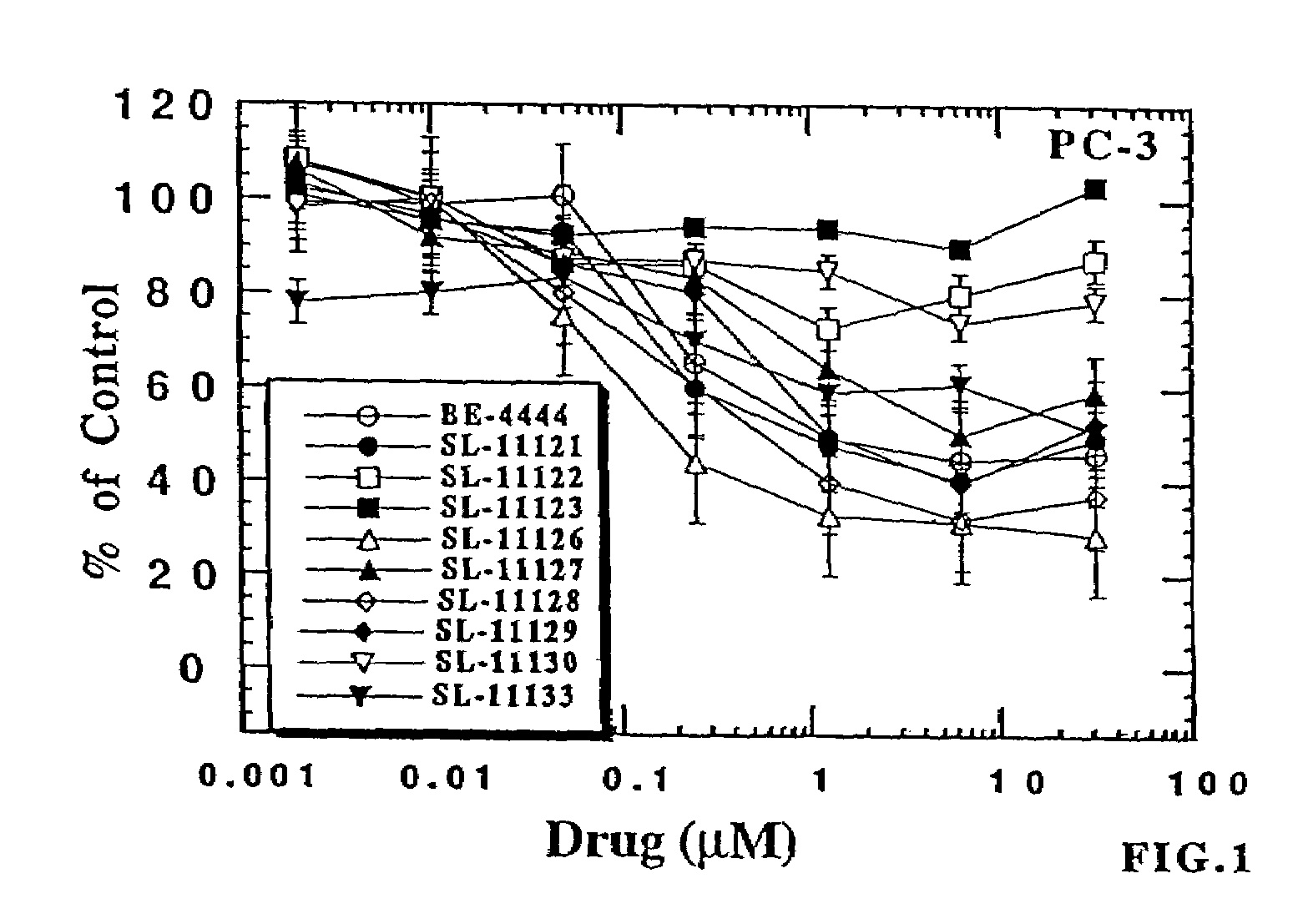
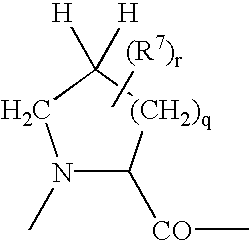
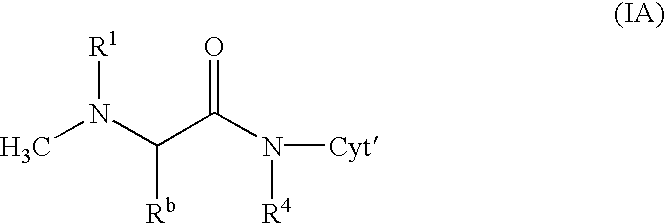
The chemotherapy of malignant tumours is greatly restricted by the generally slight differentiation of the available cytostatic agents between normal and malignant tissue. In order to achieve an improvement of the selectivity in cancer therapy, novel prodrugs have been developed from 6-hydroxy-2,3-dihydro-1H-indolene, 5-hydroxy-1,2-dihydro-3H-pyrrolo[3,2-e]indolene and 5-hydroxy-1,2-dihydro-3H-benzo[e]indolene as well as from 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]-quinolines, that may be used within the framework of the ADEP therapy (antibody directed enzyme prodrug therapy). The new prodrugs are characterised by a high difference in toxicity between the prodrug and underlying drug and by a very high efficacy of the drug. The high selectivity of the new prodrugs is probably attributed to the fact that, in the new prodrugs, a secondary halide is present in contrast to the prodrugs of a similar type previously produced by us. The direct alkylation of the DNA or RNA by the prodrugs and thus the toxicity of the prodrugs is thereby reduced. After splitting off of the glycosidic and / or acetal group on the phenolic hydroxy groups of the prodrugs, a spirocyclopropacyclohexadiene is formed which, being a highly toxic group, effects an alkylation of the DNA or RNA.
Owner:TIETZE LUTZ F
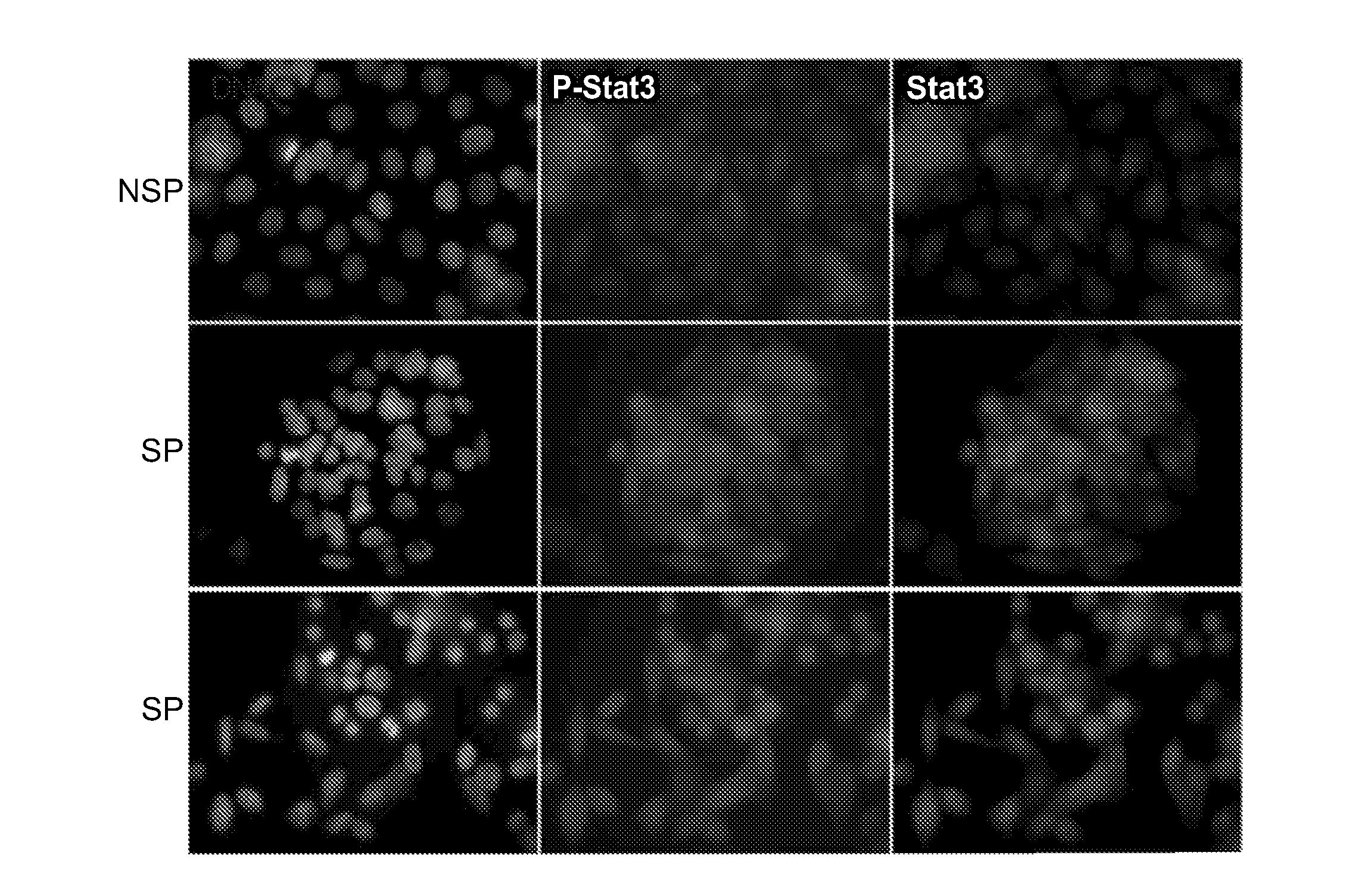
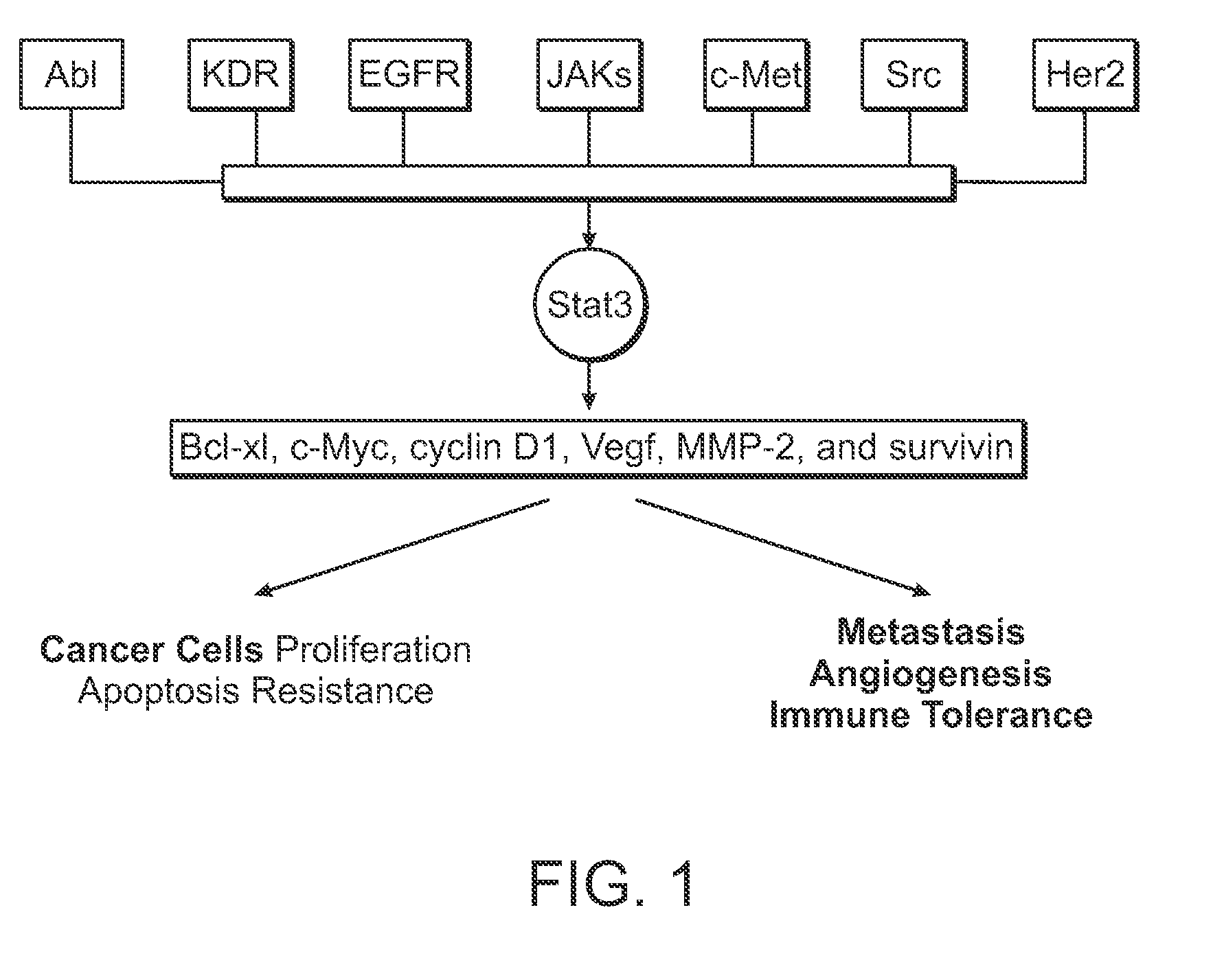
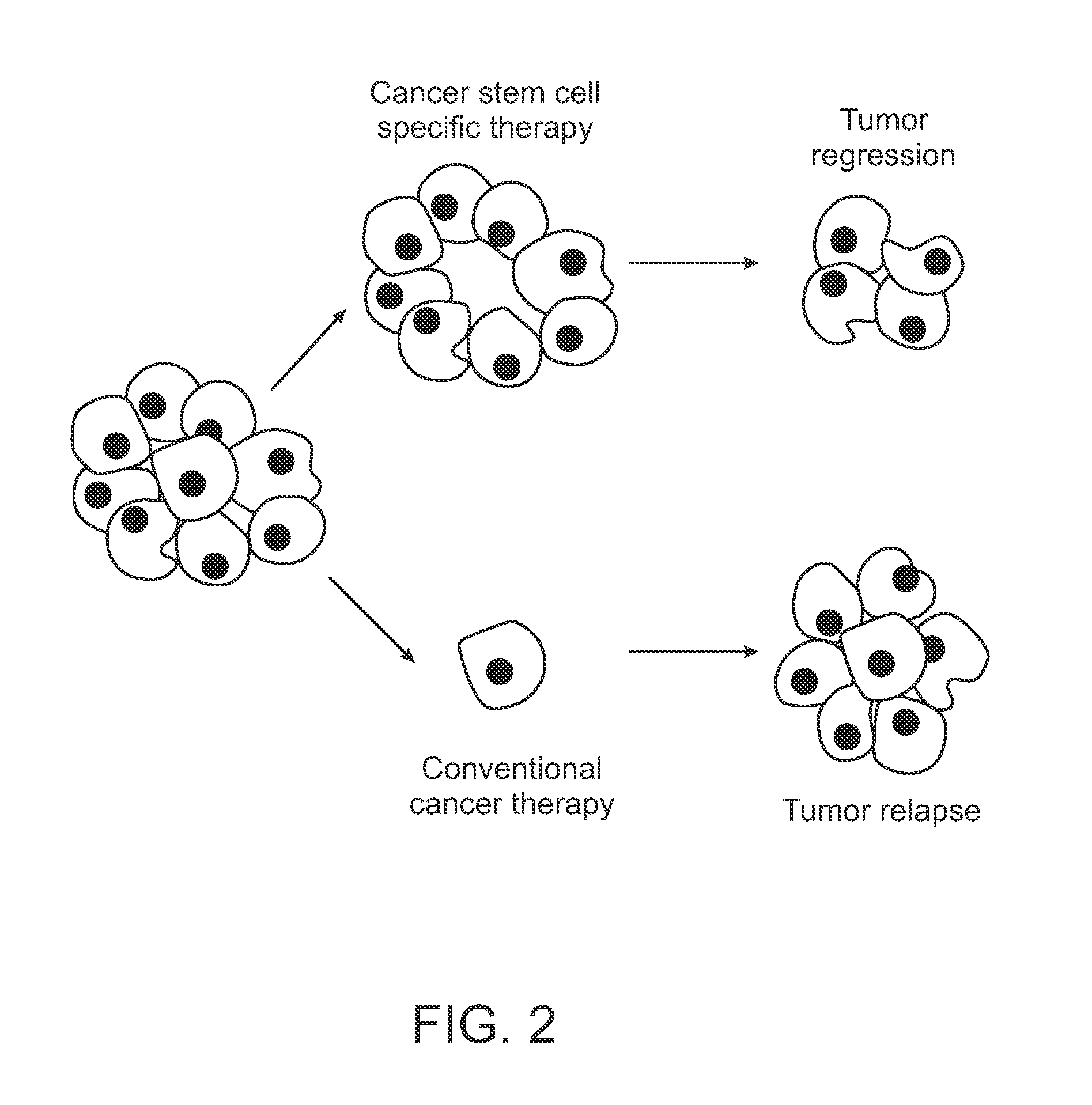
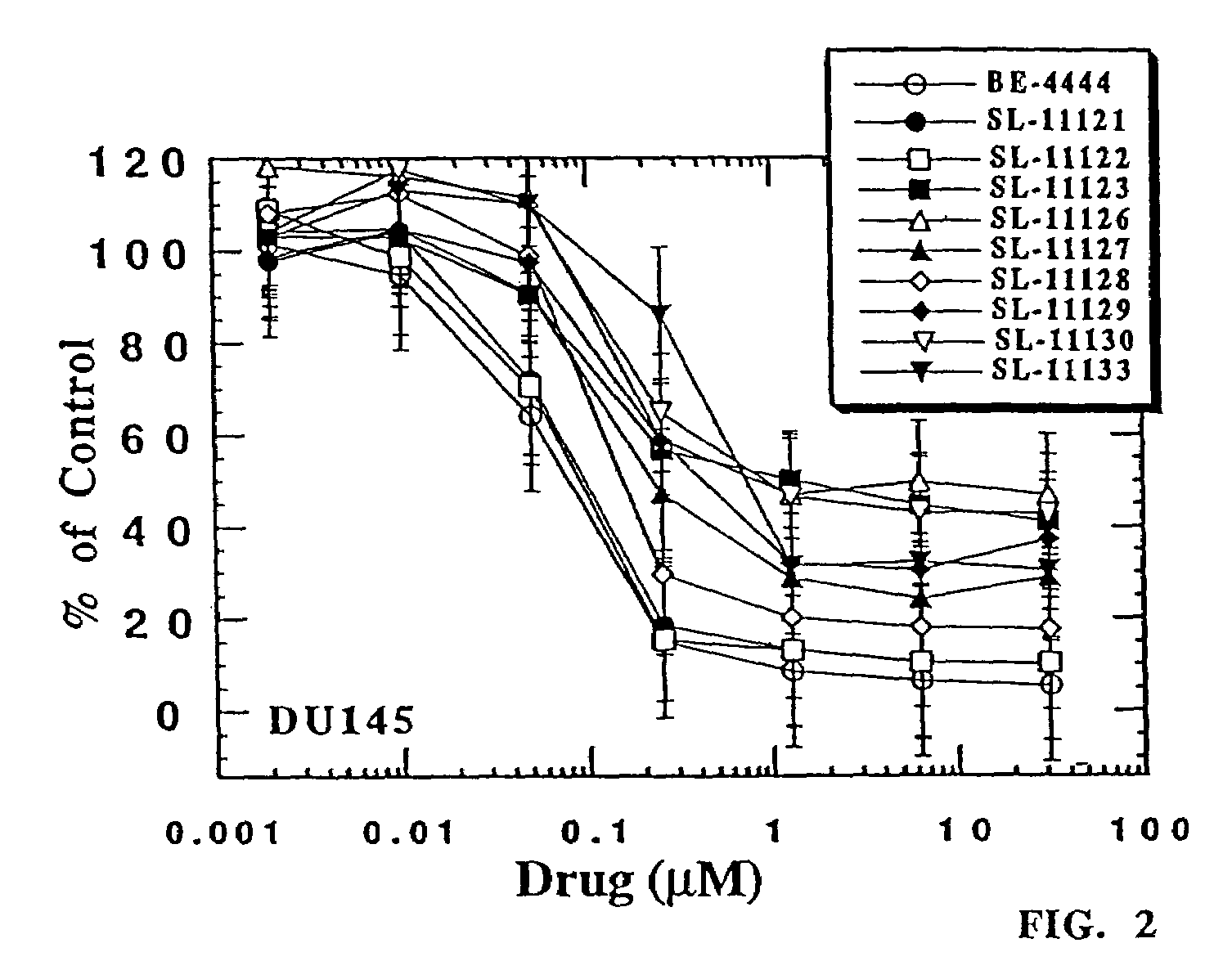
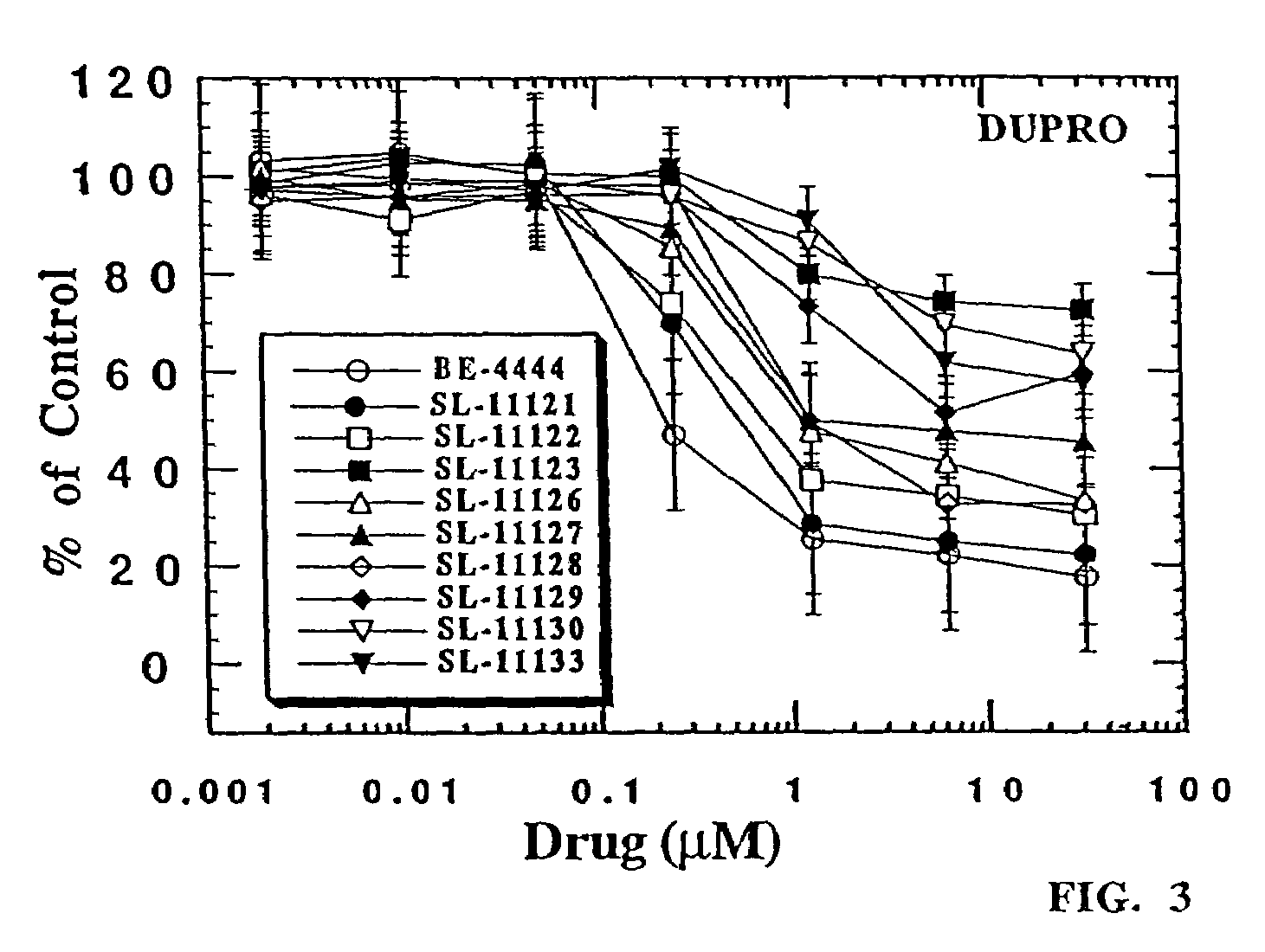
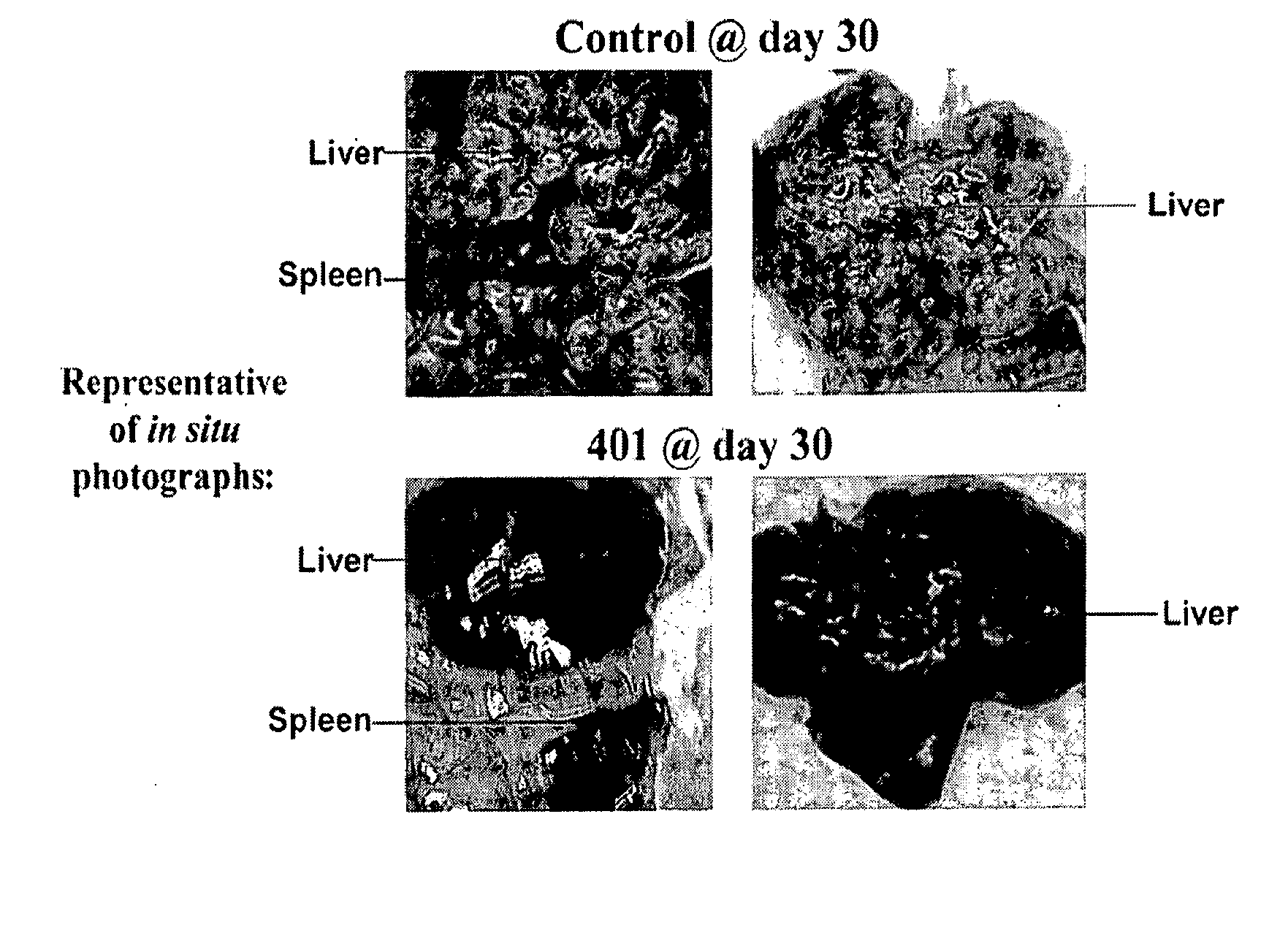
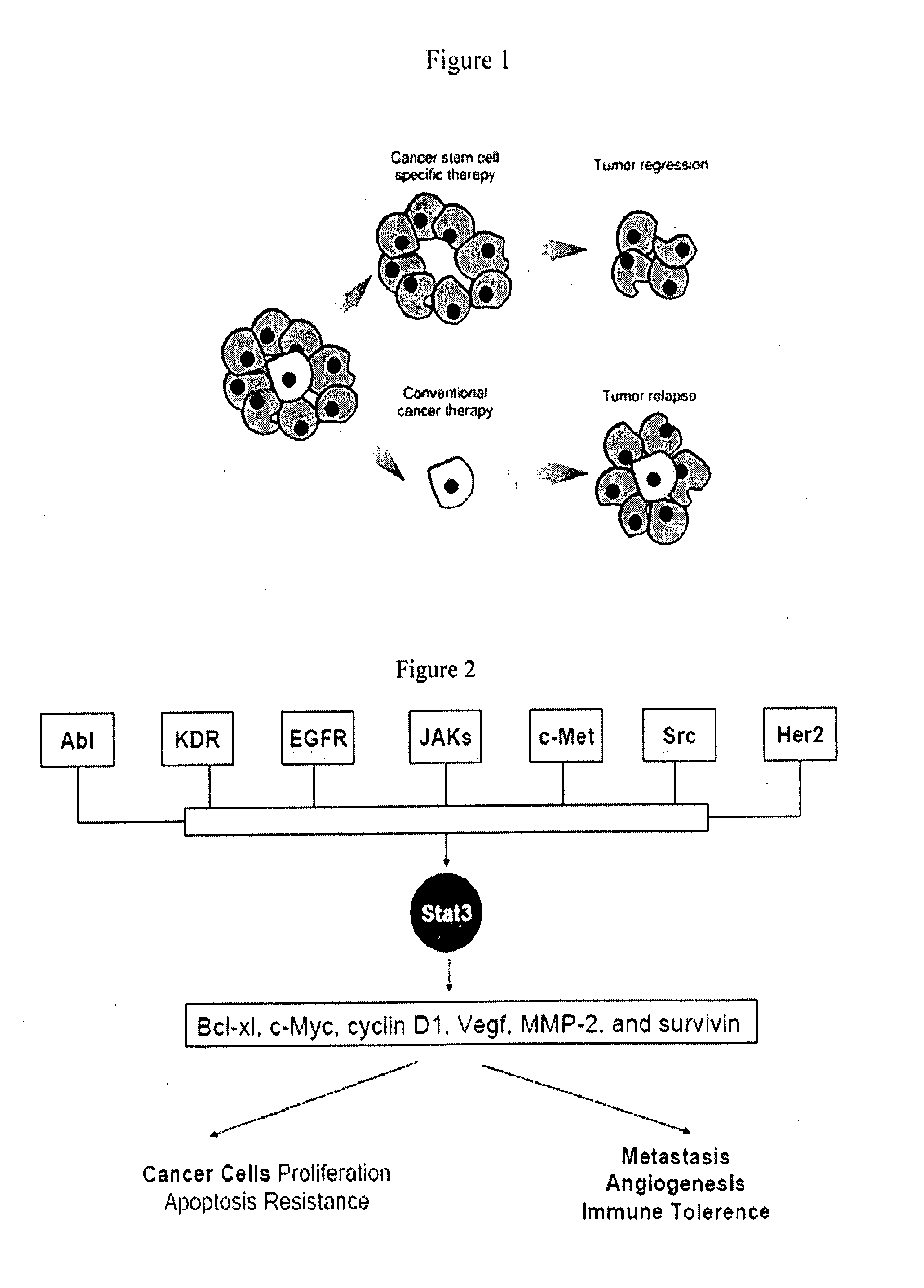
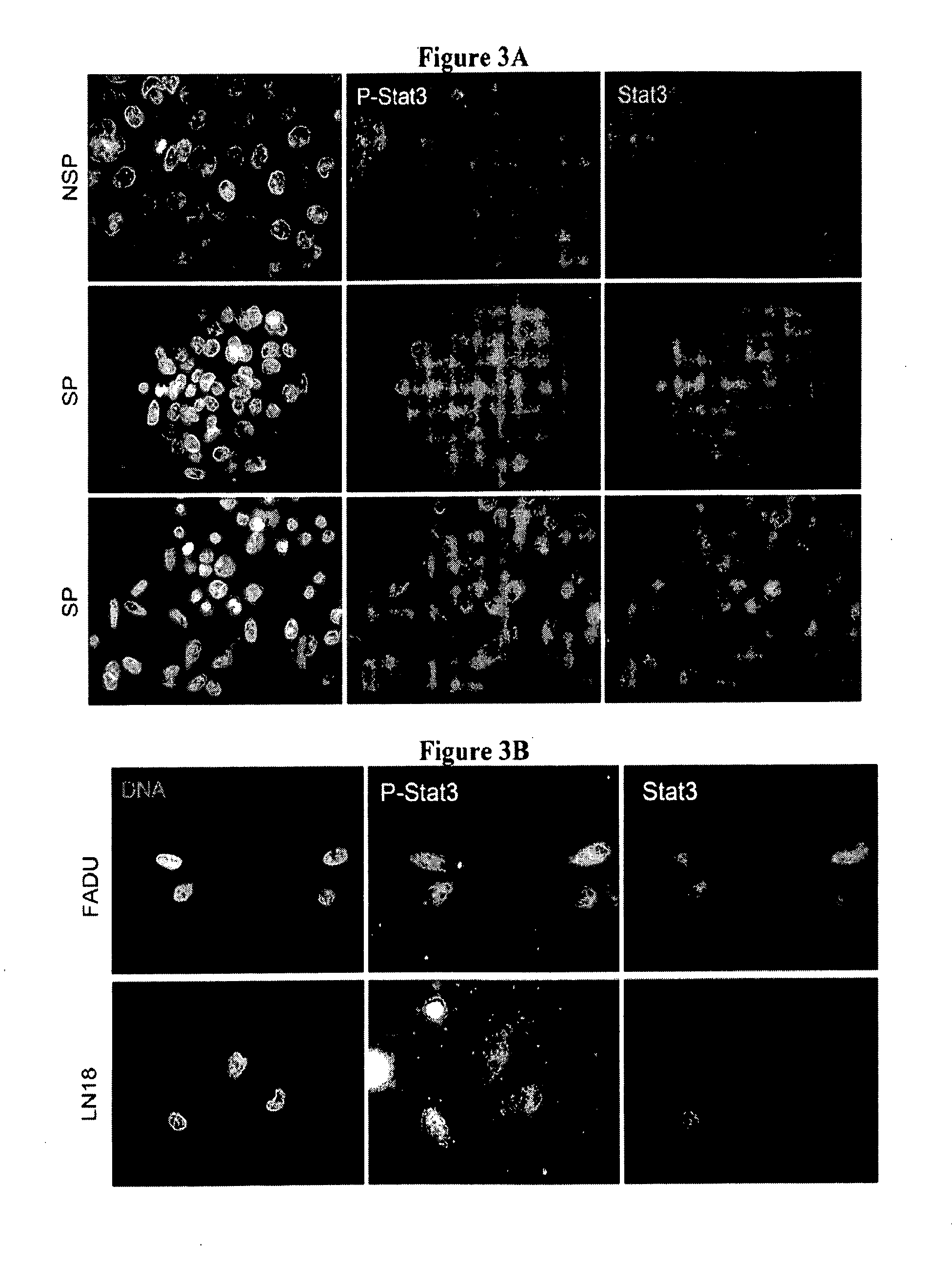
Novel stat3 pathway inhibitors and cancer stem cell inhibitors
InactiveUS20110112180A1Inhibitory activityReduced activityBiocideNervous disorderMedicinePathway activity
The present invention relates to a novel naphtho class of compounds as Stat3 pathway inhibitors and as cancer stem cell inhibitors; to methods of using such compounds to treat cancer; to methods of using such compounds to treat disorders in a mammal related to aberrent Stat3 pathway activity; to pharmaceutical compositions containing such compounds.
Owner:SUMITOMO DAINIPPON PHARMA ONCOLOGY INC
Inhibitors of kinases and cancer stem cells, and methods of preparation and use thereof
Owner:BOSTON BIOMEDICAL +1
Prodrugs for a selective cancer therapy
Owner:TIETZE LUTZ F
Stable Atomic Quantum Clusters, Production Method Thereof and Use of Same
ActiveUS20090035852A1Material nanotechnologyHeavy metal active ingredientsFluorescencePhysical chemistry
Stable atomic quantum clusters, AQCs, characterized by being composed of at least 500 metal atoms, its production process characterized by having a kinetic control and by maintaining a low concentration of reagents in the reaction medium, as well as the uses of these clusters as sensors (fluorescent, magnetic or chemical), electrocatalysts and as cytostatics and / or cytotoxics.
Owner:UNIVERSITY OF SANTIAGO DE COMPOSTELA
Conjoint therapy for treating fibrotic diseases
ActiveUS8247370B2Reduce inhibitionRelieve symptomsBiocideSenses disorderCombined Modality TherapySuppressor
The present invention relates to improved methods of treating fibrotic or fibroproliferative disorders. Conjoint therapies are provided comprising the combination of one or more fibrocyte suppressors and one or more profibrotic factor antagonists or anti-fibrotic agents.
Owner:PROMEDIOR
Cd20 therapies, cd22 therapies, and combination therapies with cd19 chimeric antigen receptor (CAR)-expressing cell
The invention provides compositions and methods for treating diseases associated with expression of CD19, e.g., by administering a recombinant T cell comprising the CD19 CAR as described herein, in combination with one or more B-cell inhibitors, e.g., inhibitors of one or more of CD1O, CD20, CD22, CD34, CD123, FLT-3, ROR1, CD79b, CD179b, or CD79a. The disclosure additionally features novel antigenbinding domains and CAR molecules directed to CD20 and CD22, and uses, e.g., as monotherapies or in combination therapies. The invention also provides kits and compositions described herein.
Owner:NOVARTIS AG +1
Fermentative preparation process for and crystal forms of cytostatics
The invention relates to a new process for concentrating epothilones in culture media, a new process for the production of epothilones, a new process for separating epothilones A and B and a new strain obtained by mutagenesis for the production of epothilones, as well as aspects related thereto. New crystal forms of epothilone B are also described.
Owner:NOVARTIS AG
Biomarkers for cancer stem cells and related methods of use
Novel methods of classifying subjects as candidates for treatment with a cancer associated mesenchymal cell, tumor initiating cancer cell, or cancer stem cell inhibitor treatment and subsequent administration of the cancer associated mesenchymal cell, tumor initiating cancer cell, or cancer stem cell inhibitor are disclosed within.
Owner:VERASTEM
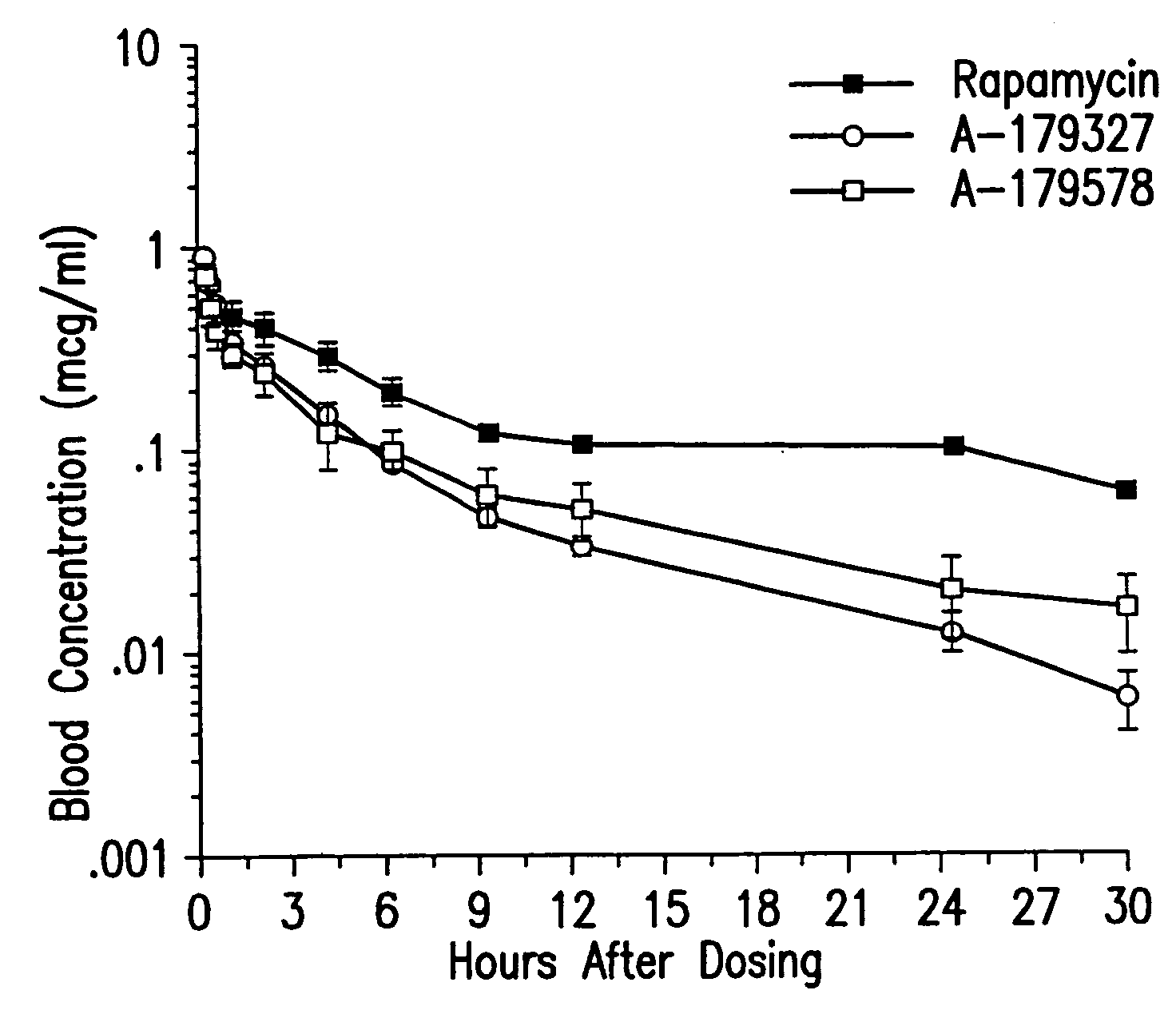
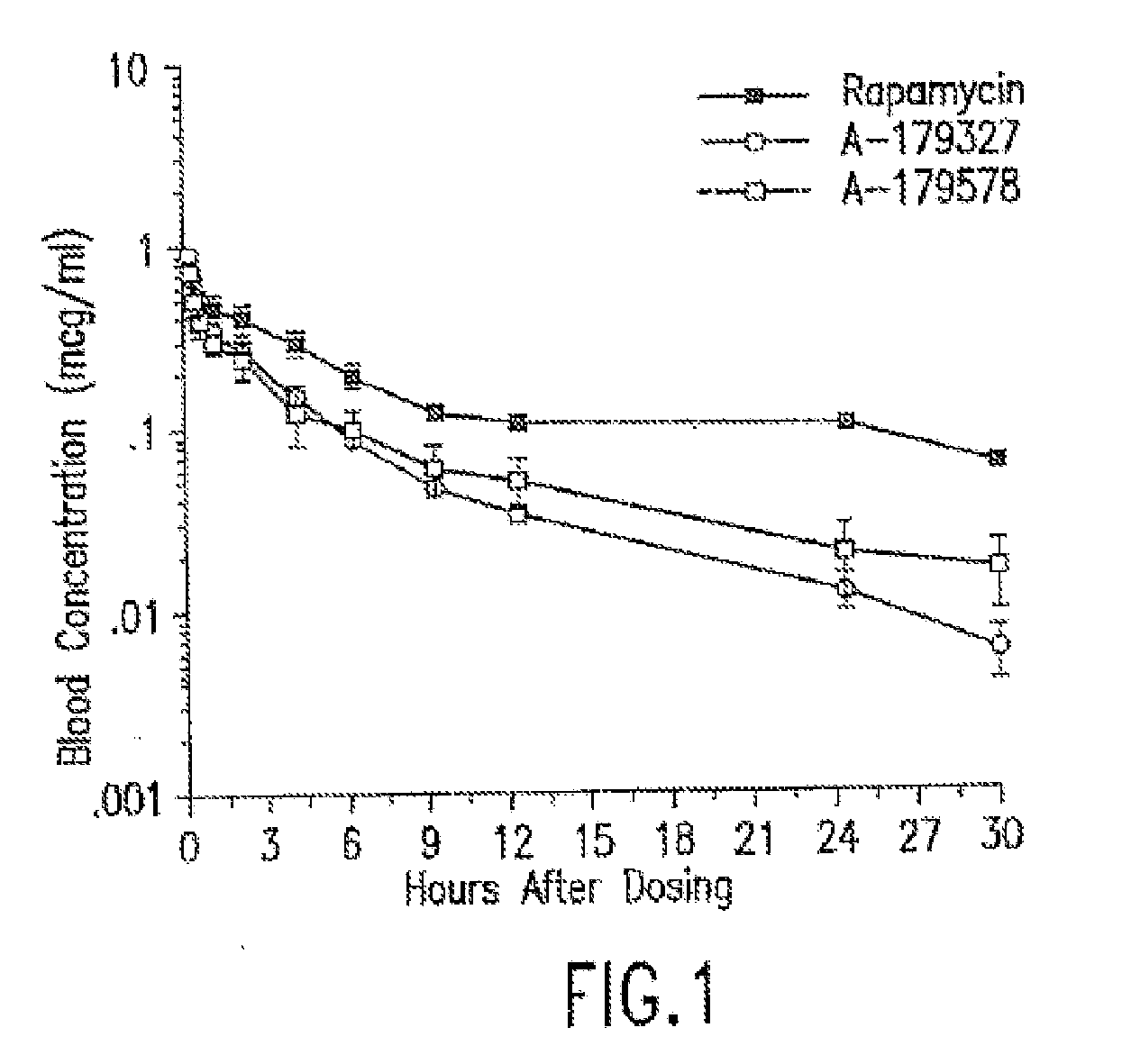
Medical devices containing rapamycin analogs
InactiveUS20060198870A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsAnti plateletAntiproliferative Agents
A medical device comprising a supporting structure having a coating on the surface thereof, the coating containing a therapeutic substance, such as, for example, a drug. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to, This drug can be used in combination with a drug selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
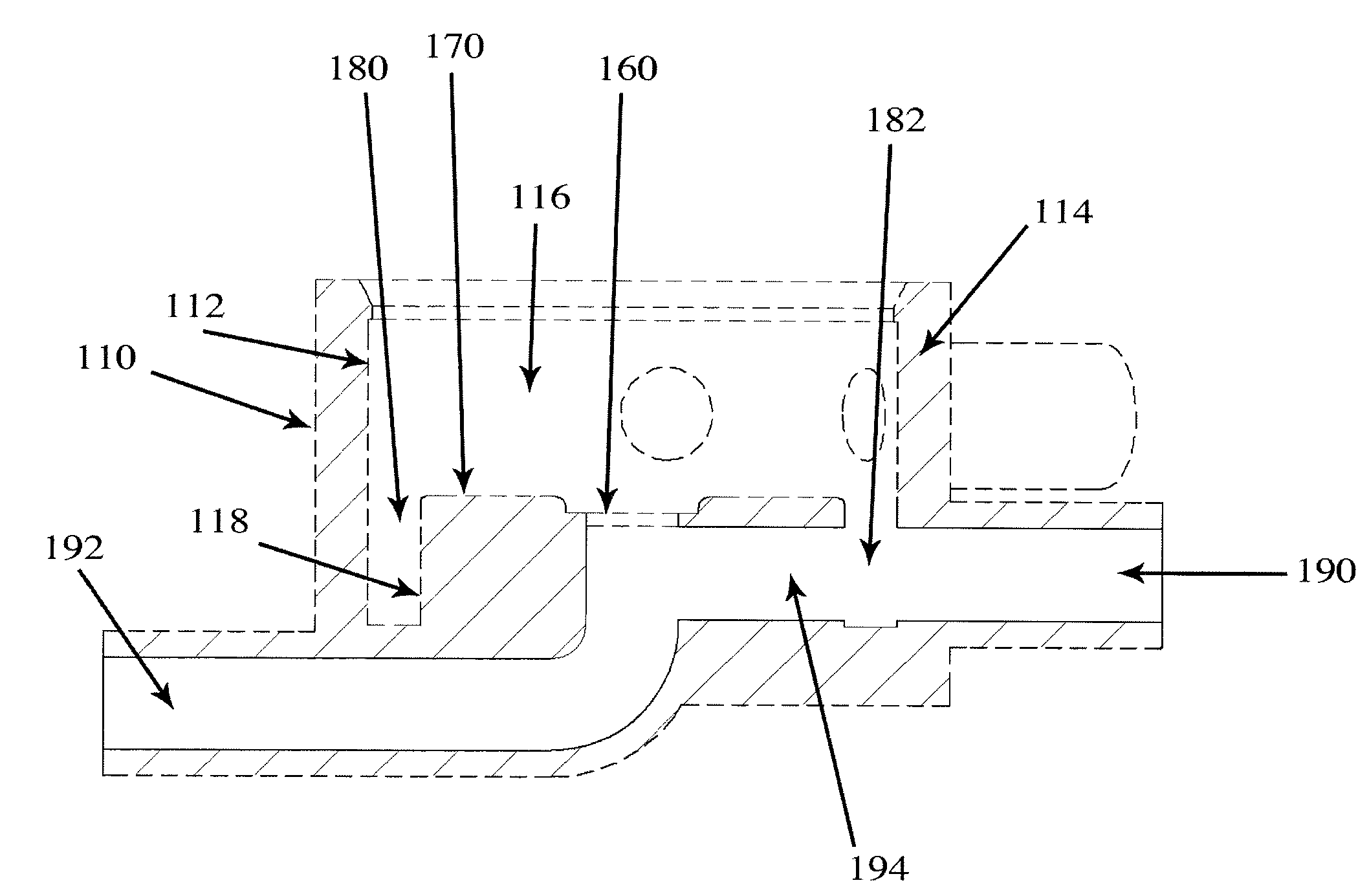
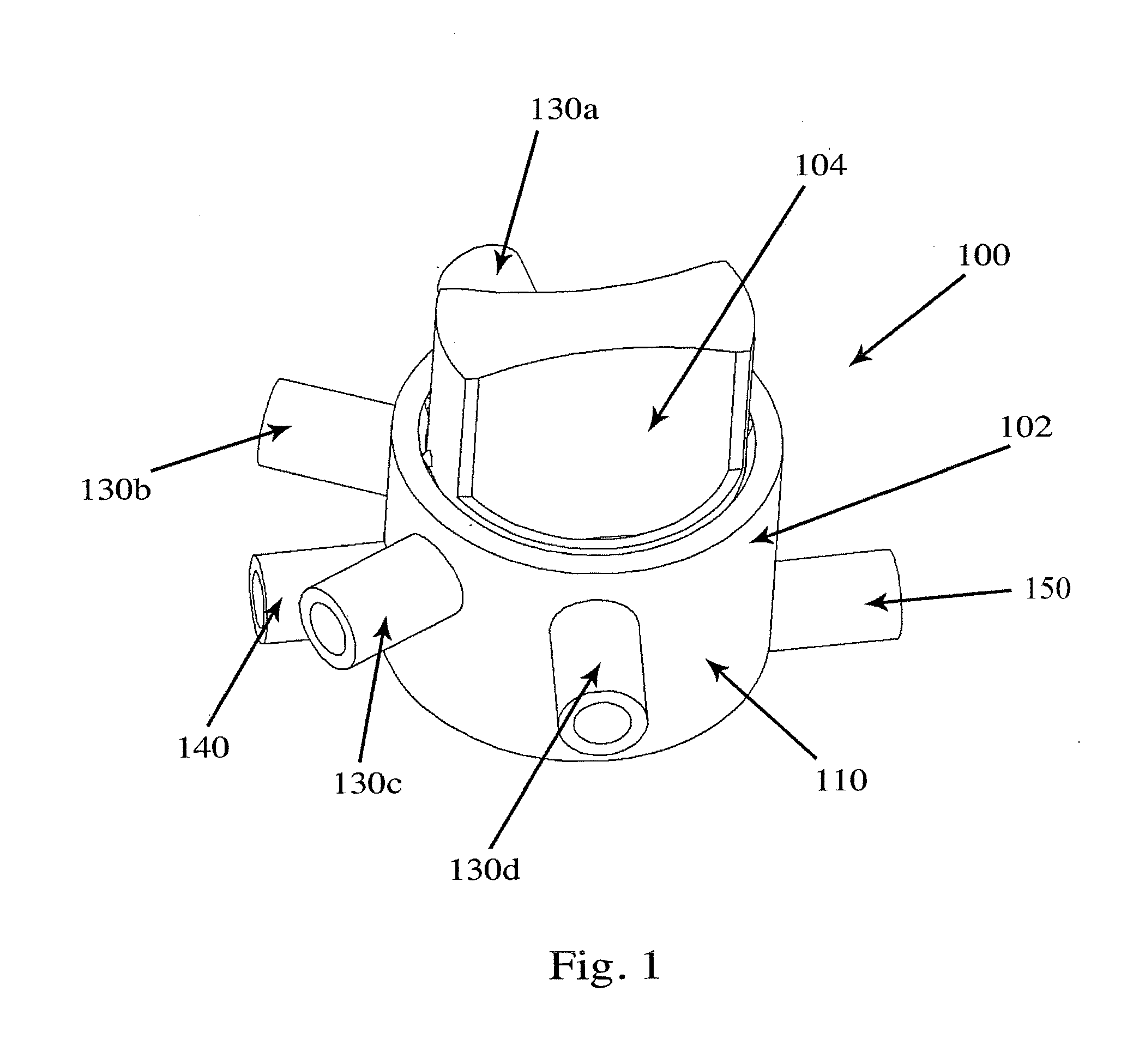
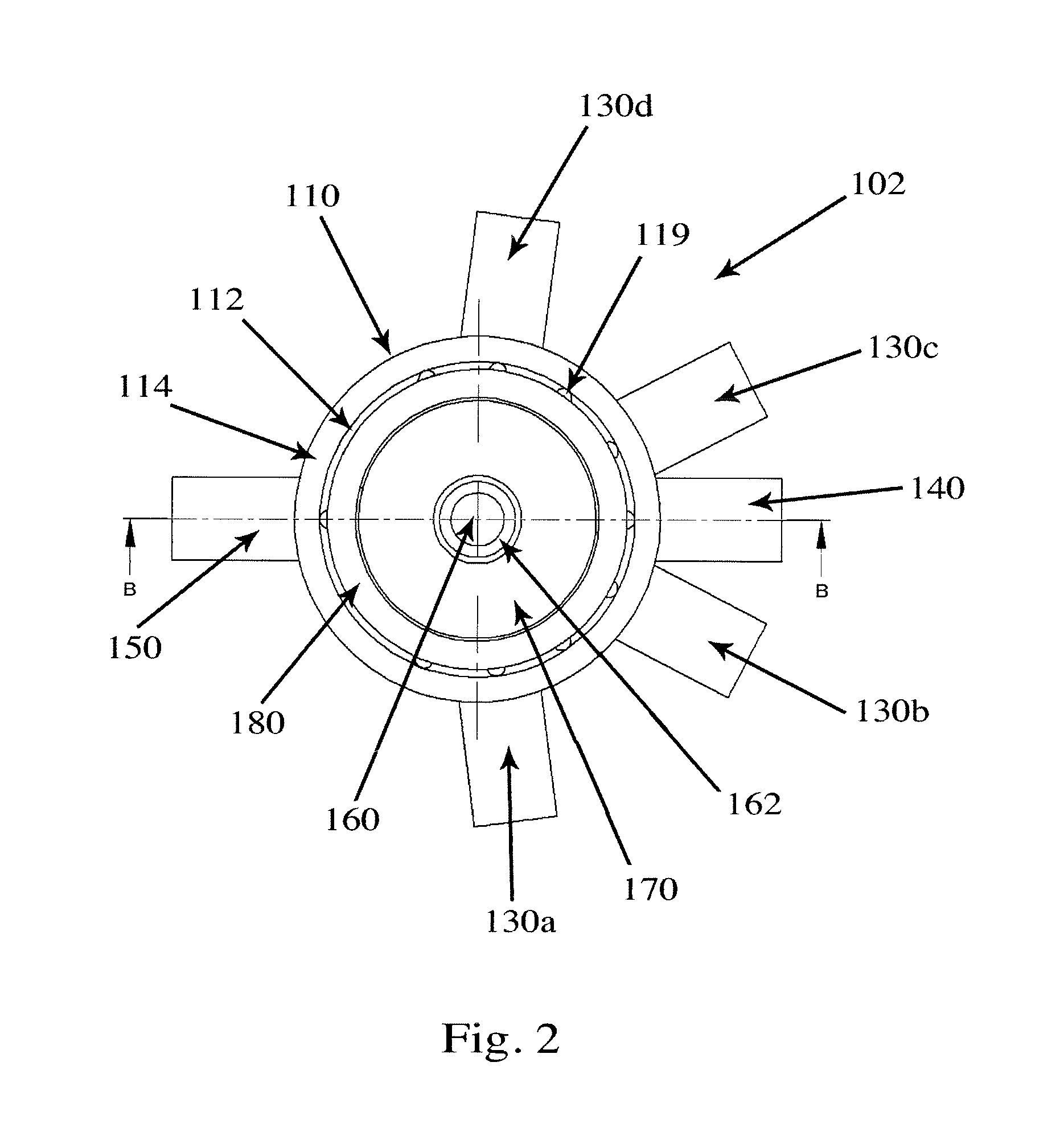
Valve for administration of a plurality of drug fluids
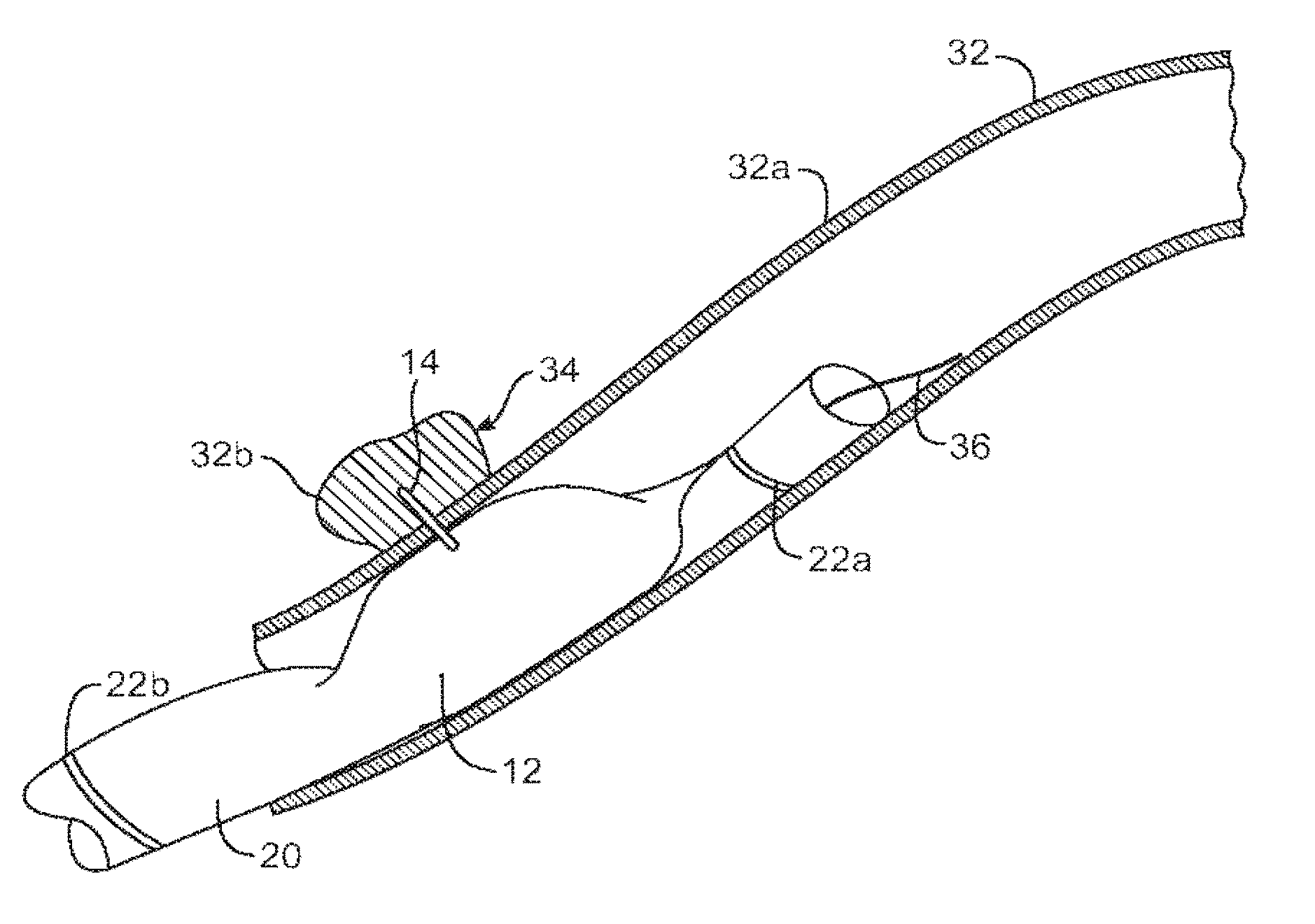
ActiveUS20140346386A1Simple and cheap to manufactureAssembly of valve simple and cheapPlug valvesInfusion devicesMedicineCytostatic agents
In accordance with the present inventive concept, there is provided a valve for administration of a plurality of drug fluids, such as cytostatics. The valve comprises: a housing having a plurality of circumferentially distributed primary inlets for receiving a respective one of the drug fluids and a secondary inlet for receiving a secondary fluid, such as a neutral fluid, an outlet, and a valve member arranged in the housing. The housing has a plurality of primary valve positions in each of which an associated one of the primary inlets is connected to the outlet, and a plurality of intermediary valve positions in each of which the secondary inlet is connected to the outlet. Moreover, the valve member h a outer surface sealingly engaging an inner surface of the housing, such that the primary and secondary inlets are sealingly connected to openings arranged in the outer surface of the valve member in each of the primary and intermediary valve positions, respectively. Figure for publication.
Owner:CYT0365 AB
Aryl urea compounds in combination with other cytostatic or cytotoxic agents for treating human cancers
Owner:CARTER CHRISTOPHER A +6
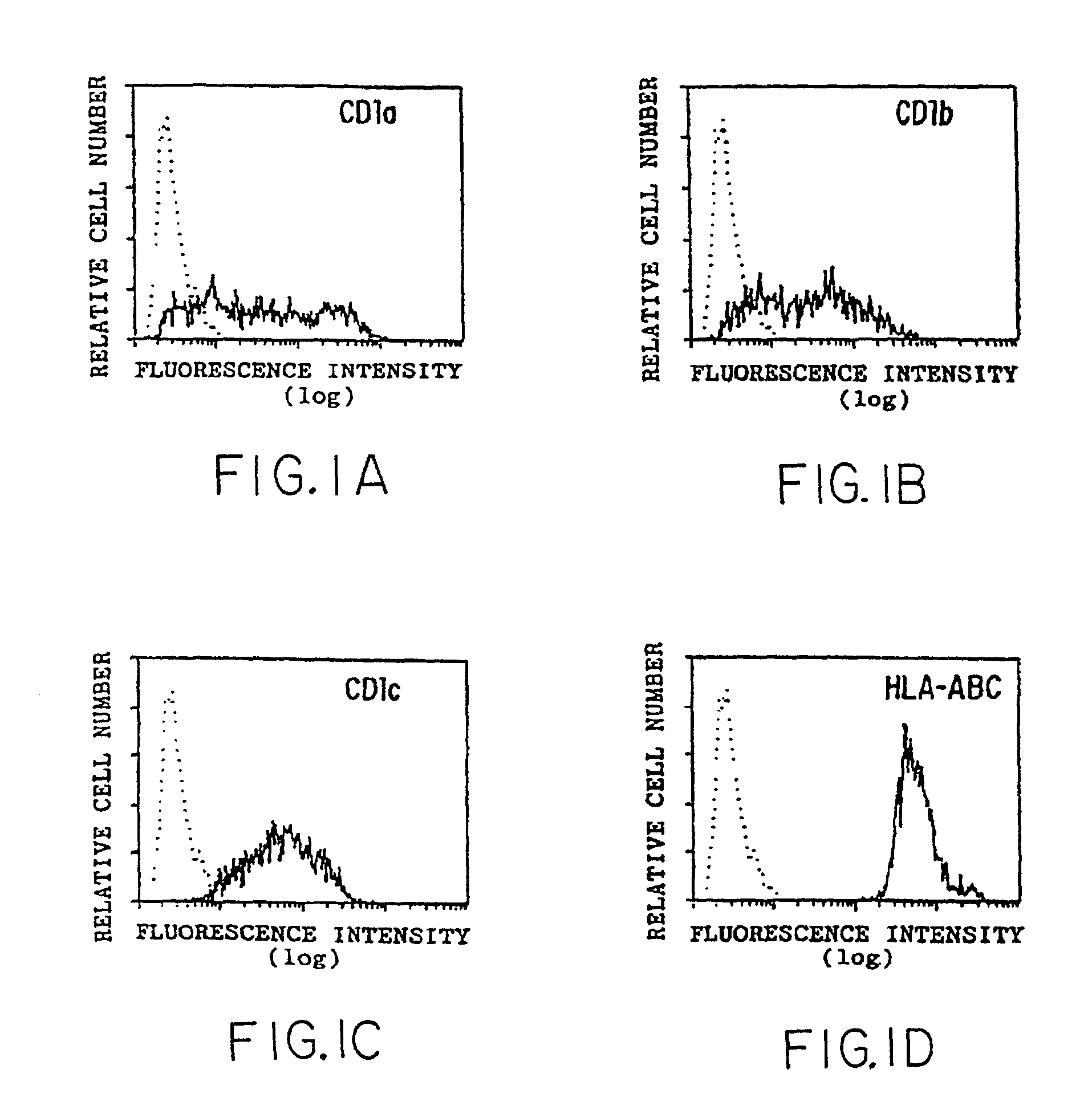
Presentation of hydrophobic antigens to T-cells by CD1 molecules
Provided are CD-1 presented antigens, compositions, cells, inhibitors and methods relating to the use of hydrophobic antigen presentation by CD1 molecules, including: methods for detecting the presence of a CD1-presented hydrophobic antigen in a sample; methods for isolating such CD1-presented antigens and the isolated antigens; vaccines containing CD1-presented antigens and vaccination methods; methods of blocking CD1 antigen presentation; methods of identifying and / or isolating CD1 blocking agents and the isolated CD1 blocking agents; methods of inducing CD1 expression; and T-cells for use in the methods disclosed herein.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
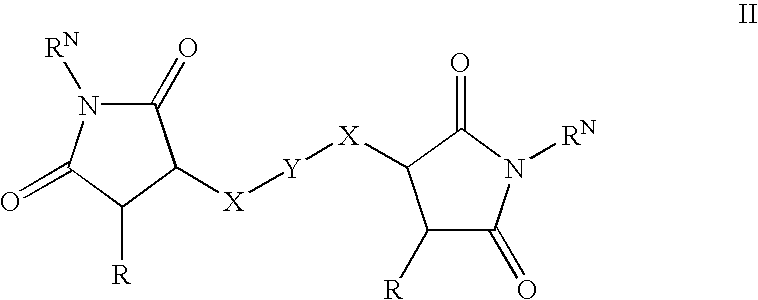
Succinimide and maleimide derivatives and their use as topoisomerase II catalytic inhibitors
InactiveUS20030032625A1Avoid cytotoxicityGood effectBiocideOrganic chemistryDNA underwindingTopoisomerase-II Inhibitor
Maleimide and succinimide derivatives were found to be effective topoisomerase II catalytic inhibitors. Due to this property, the maleimide and succinimide derivatives were investigated for their use as cytostatic agents and thus in the treatment of cancer. The compounds of the invention can be used in combination treatments with other cytostatic agents, such as topoisomerase II poisons. The maleimide and succinimide derivatives, due to their effective topoisomerase II catalytic inhibitory activity, are also useful as extravasation agents, such as upon administration of a topoisomerase II poison.
Owner:TOPOTARGET AS
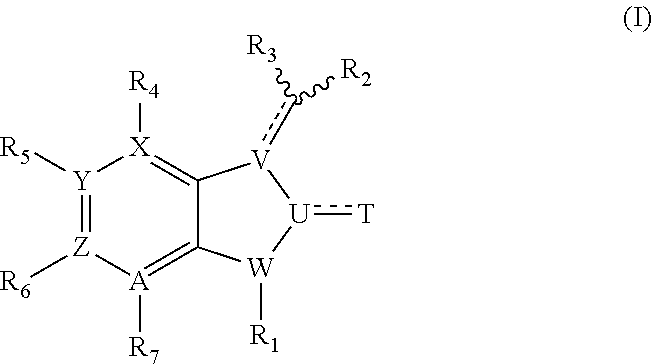
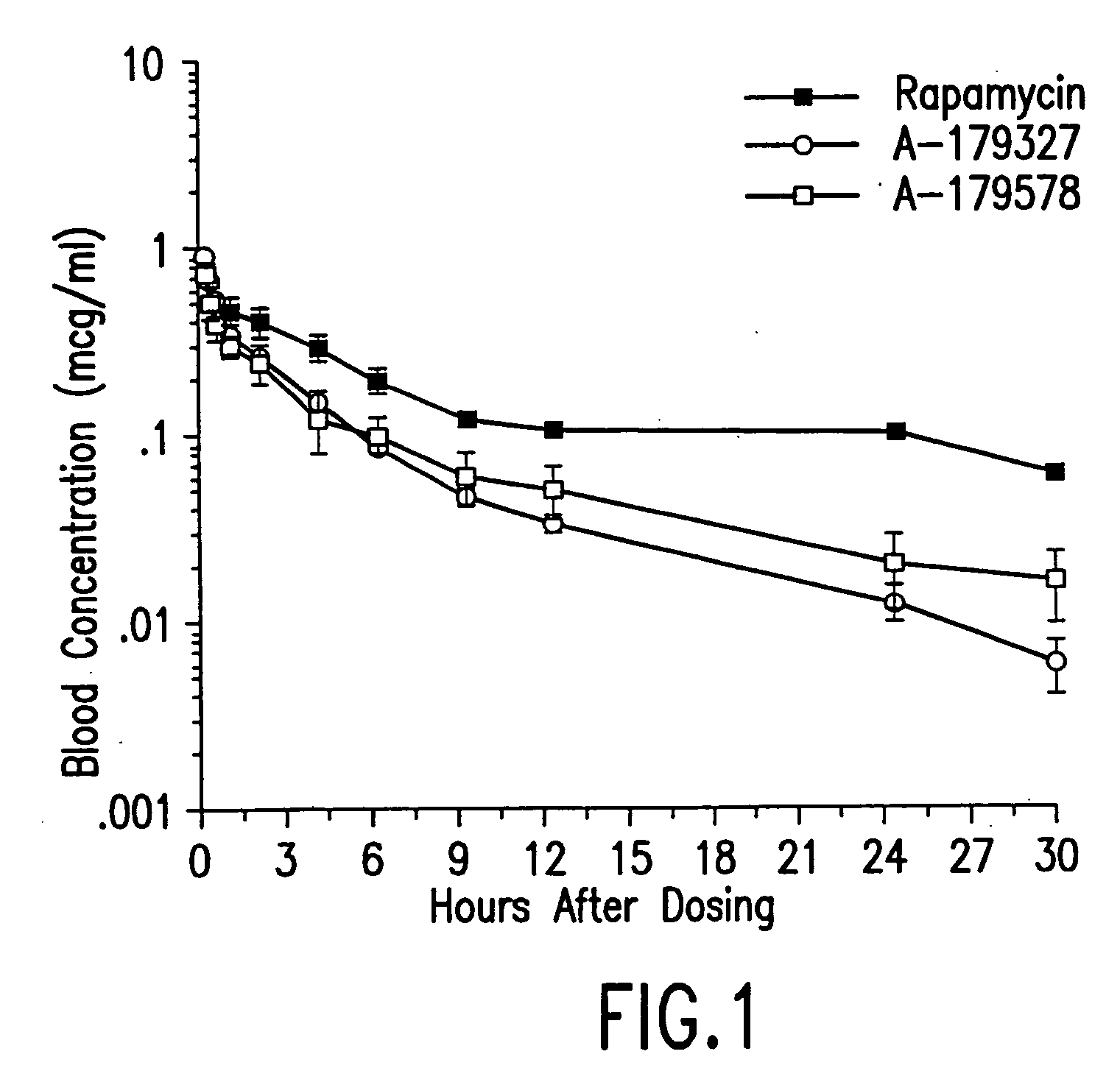
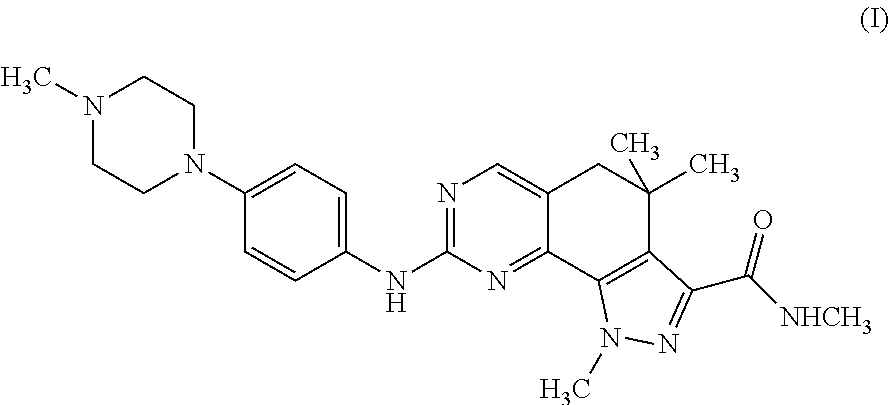
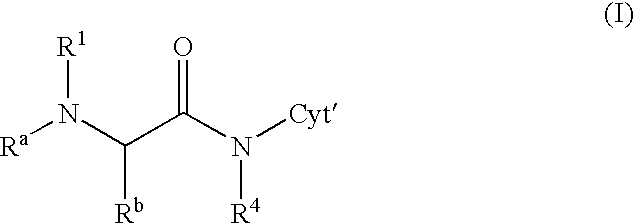
Medical Devices Containing Rapamycin Analogs
InactiveUS20080153790A1Reduce probabilityReduces restenosis in vasculatureBiocideAntimycoticsAnti plateletCytostatic drugs
A medical device comprises a supporting structure capable of containing or supporting a pharmaceutically acceptable carrier or excipient, which carrier or excipient may contain one or more therapeutic agents or substances, with the carrier preferably including a coating on the surface thereof, and the coating containing the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. Drugs that are suitable for use in this invention include, but are not limited to drugs of Formula (I). The drugs of Formula (I) can be used in combination with another drug including those selected from anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, cytostatic drugs, or combinations of these drugs.
Owner:ABBOTT LAB INC
Maintenance of Bronchial Patency by Local Delivery of Cytotoxic, Cytostatic, or Anti-Neoplastic Agent
InactiveUS20150119850A1Keep openLimit recurrent bronchial occlusionOrganic active ingredientsBiocideCytotoxicitySubmucosa
Methods for maintaining patency in a bronchus of a patient are presented. A catheter is positioned within the bronchus. A target region of one or more of a bronchial wall, submucosa, media, and adventitia is punctured at or adjacent a location of a debulked bronchial carcinoma with an injection needle disposed on a distal end of the catheter. Such puncturing is achieved by expanding a balloon disposed on the distal end of the catheter. The balloon is comprised of at least two materials of different elastic modulus, which allows for a flexible but relatively non-distensible, unfolding component of the balloon as well as an elastomeric, inflatable component of the balloon. Through the injection needle, an amount of cytotoxic, cytostatic, or anti-neoplastic agent is delivered to the target region. The delivered amount is effective to limit by a therapeutically beneficial amount recurrent bronchial occlusion due to recurrence of the bronchial carcinoma.
Owner:MERCATOR MEDSYST
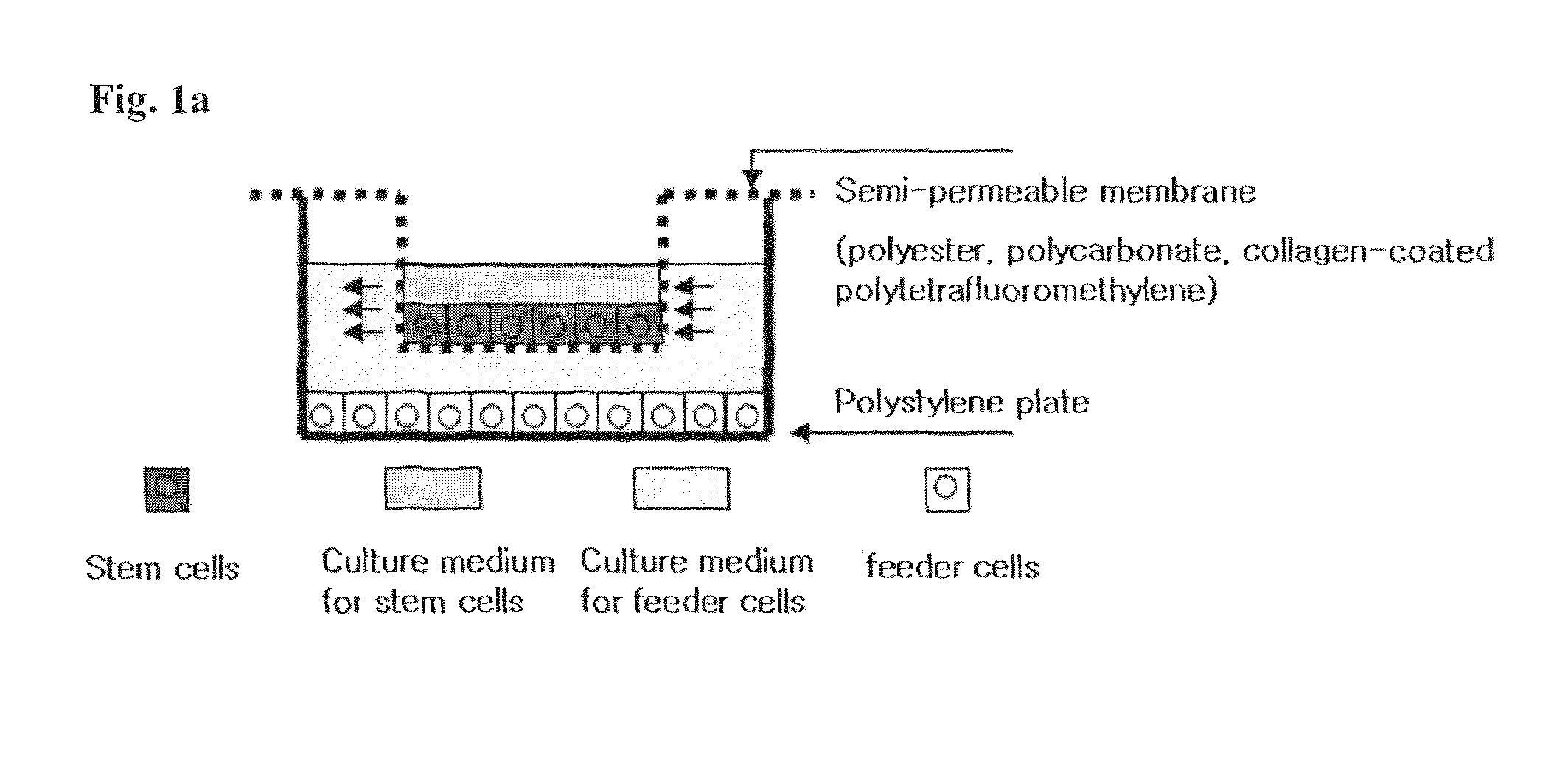
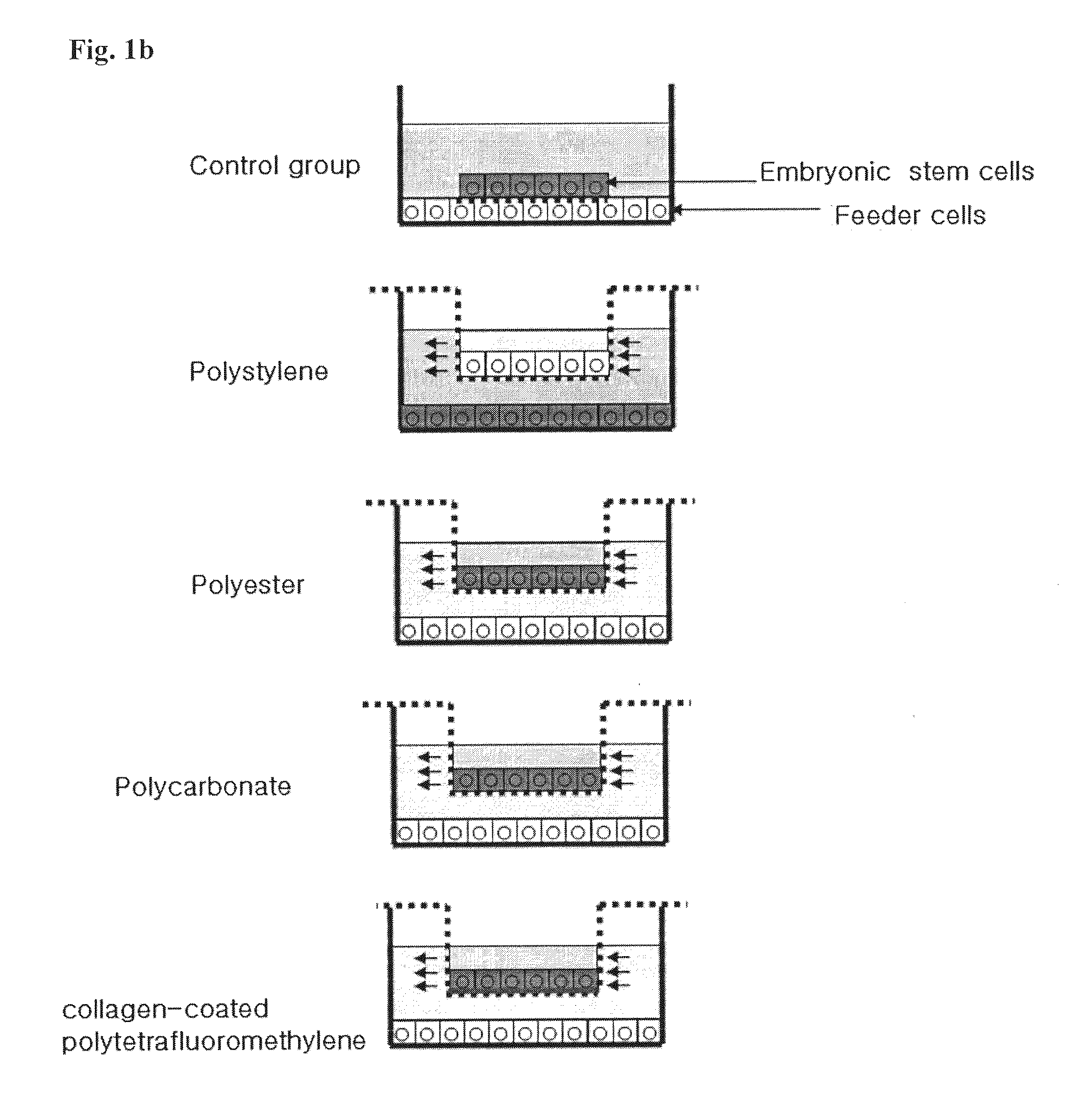
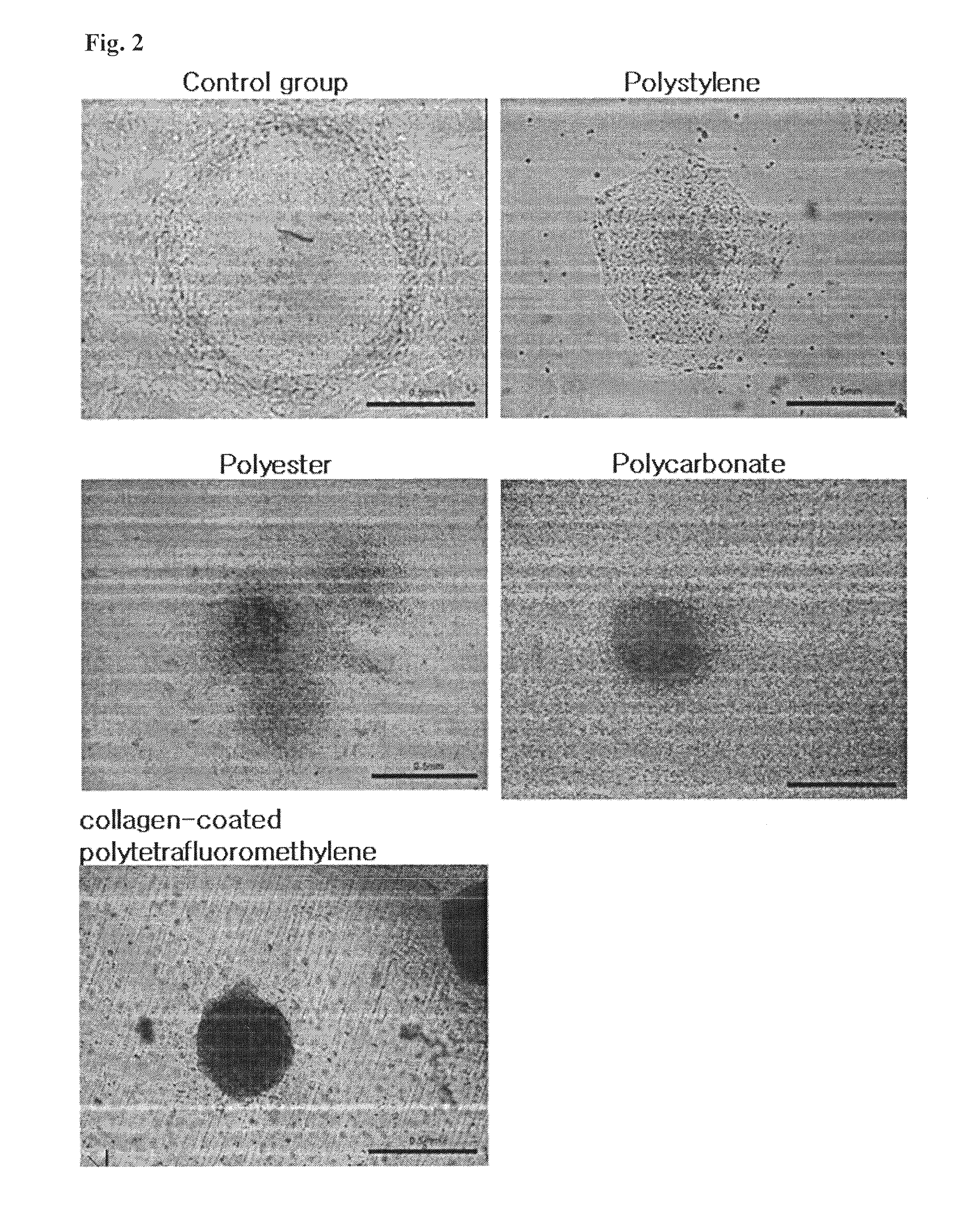
Method for co-culture of human embryonic stem cells and fibroblast feeder cells using a polyester membrane
Owner:MODERN CELL & TISSUE TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
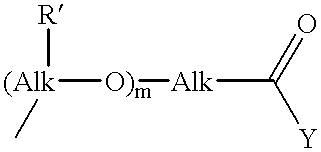
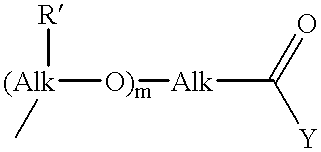

![Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy](https://images-eureka.patsnap.com/patent_img/fa862af6-46c5-47d9-9bd3-8a3b0f48399d/US20040033962A1-20040219-C00001.png)
![Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy](https://images-eureka.patsnap.com/patent_img/fa862af6-46c5-47d9-9bd3-8a3b0f48399d/US20040033962A1-20040219-C00002.png)
![Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy Novel prodrugs von 6-hydroxy-2,3-dihydro-1h-indoles, 5-hydroxy-1,2-dihydro-3h-pyrrolo[3,2-e]indoles and 5-hydroxy-1,2-dihydro-3h-benzo(e)indoles as well as of 6-hydroxy-1,2,3,4-tetrahydro-benzo[f]quinoline derivatives for use in selective cancer therapy](https://images-eureka.patsnap.com/patent_img/fa862af6-46c5-47d9-9bd3-8a3b0f48399d/US20040033962A1-20040219-C00003.png)