Cd20 therapies, cd22 therapies, and combination therapies with cd19 chimeric antigen receptor (CAR)-expressing cell
A CD22 and cell technology, applied in the direction of receptor/cell surface antigen/cell surface determinant, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, animal cells, etc., can solve incurable and clinically effective Sexual difficulties, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1827] Example 1: CD19 CAR T cells for the treatment of multiple myeloma
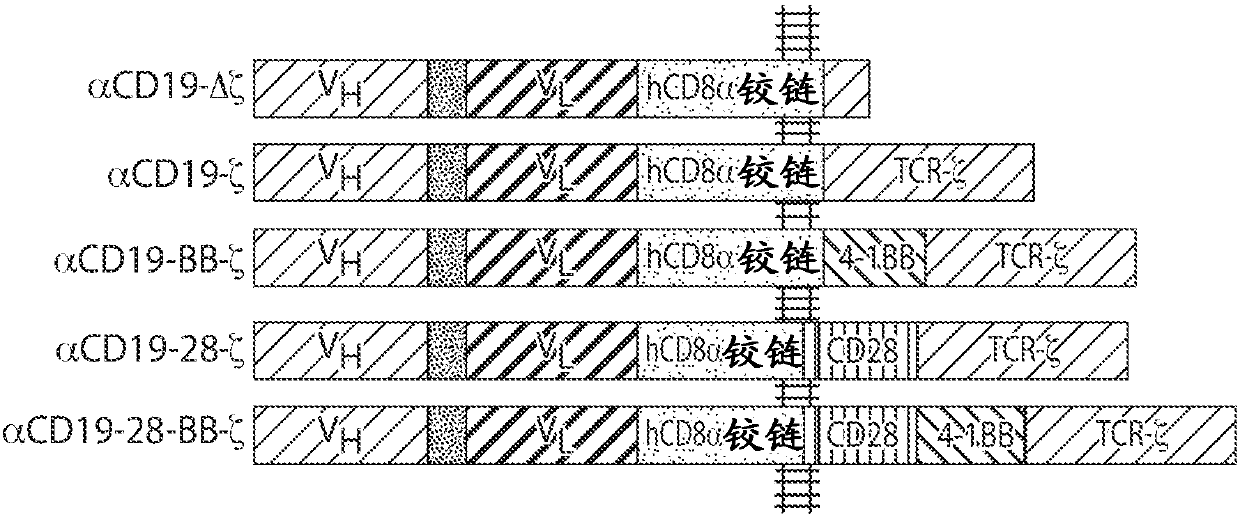
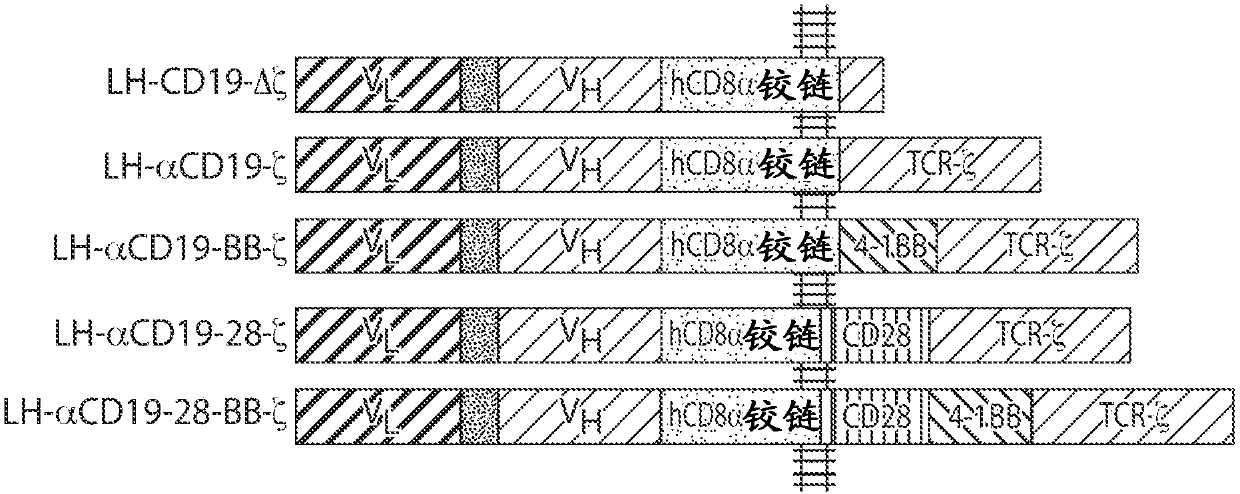
[1828] Even with current chemotherapy regimens targeted therapy and autologous stem cell transplantation, myeloma is considered incurable. This example describes the treatment of multiple myeloma (MM) with CD19-directed autologous T cells with a chimeric antigen receptor (lentivirus / CD19:4-1BB:CD3zeta; also known as "CART19" or CTL019). This example demonstrates that CAR therapy targeting CD19 has the potential to establish deep long-term durable responses based on targeting myeloma stem cells and / or tumor cells expressing very low (undetectable by most methods) levels of CD19.
[1829] In treating patients with aggressive secondary plasma cell leukemia, we found that CART19 administered two days after salvage autologous stem cell transplantation resulted in rapid clearance of plasma cell leukemia, with a very good fraction in patients undergoing multiple lines of chemotherapy reaction. The patient wa...
Embodiment 2
[1915] Example 2: CAR19T cell therapy for Hodgkin's lymphoma
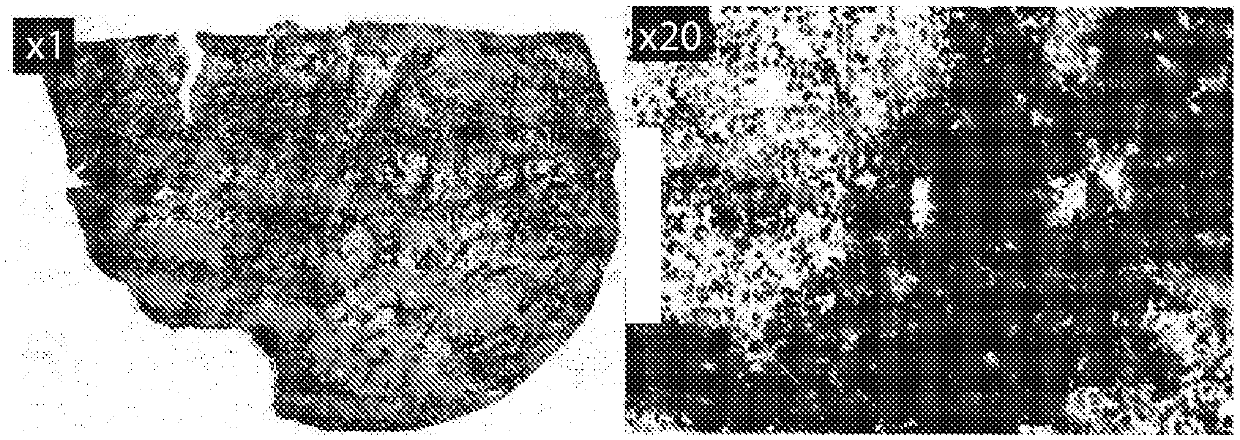
[1916] CAR19T cell therapy can also be used to treat Hodgkin's lymphoma (HL). Hodgkin's lymphoma is characterized by the presence of malignant Hodgkin Reed-Sternberg (HRS) cells derived from clonal germinal center B cells. Several factors suggest the efficacy of CAR19T cell therapy for HL. CD19 staining of HL tumors revealed CD19-expressing (CD19+) cells within the tumor and tumor microenvironment ( figure 2 ). A study showed that clonal B cell populations expressing CD19 (CD20 + CD27 + ALDH + ) is responsible for the generation and maintenance of Hodgkin's lymphoma cell lines and also circulates in the blood of most HL patients (Jones et al., Blood, 2009, 113(23):5920-5926). This clonal B cell population is also thought to cause or contribute to the generation of malignant HRS cells. Thus, CART19 treatment will deplete the B cell population that contributes to tumorigenesis or maintenance of tumor cells. ...
Embodiment 3
[1922] Example 3: A Subset of Non-Responders in CLL Patients Shows Increased Expression of Immune Checkpoint Inhibitor Molecules
[1923] In the present study, the expression of immune checkpoint inhibitor molecules such as PD-1, LAG3 and TIM3 was assessed in clinically manufactured CART19 cells from 34 CLL patients. The response of this cohort to CART19 is known, so the correlation between response and biomarker expression patterns can be assessed.
[1924] Prepared CART19 cells from CLL patients with different responses to CART therapy were analyzed by flow cytometry to determine the expression of CAR and immune checkpoint inhibitor molecules PD-1, LAG3 and TIM3. CART19 cells were derived from: healthy donors (HD) (n=2); CLL patients (n=5) who responded to CART therapy (CR); CLL patients who partially responded to CART therapy (PR) (n=8); did not respond to CART CLL patients treated (NR) (n=21). Cells were stained with fluorescently labeled antibodies that specifically rec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com