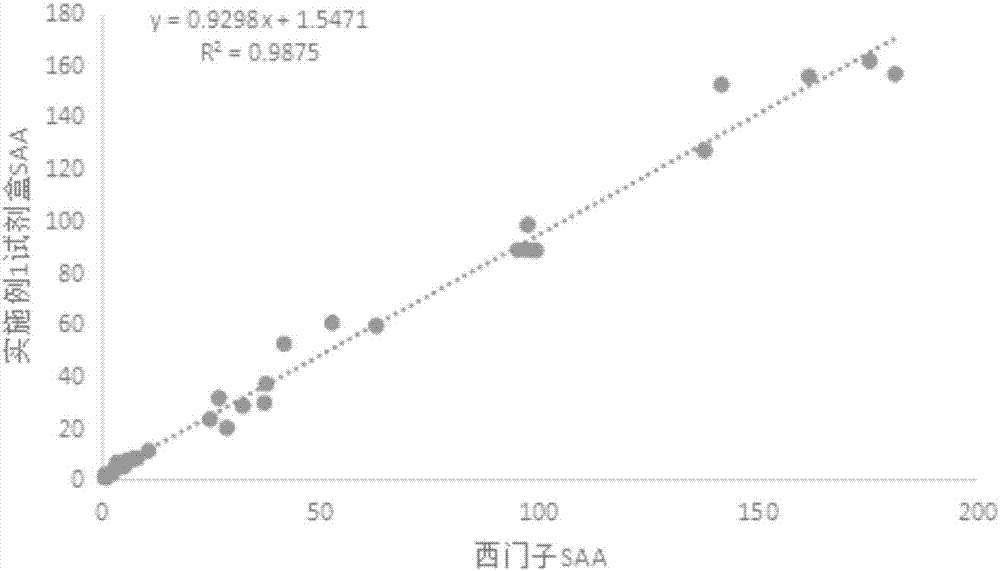
Immunofluorescence chromatography kit for quantitative detection of SAA (serum amyloid A), CRP (C-reactive protein) and PCT (procalcitonin) and preparation method of immunofluorescence chromatography kit
A quantitative detection and immunofluorescence technology, applied in measurement devices, analytical materials, instruments, etc., can solve the problems of difficult to meet quantitative detection requirements, poor sensitivity, low accuracy, etc., and improve the accuracy of poor accuracy, high sensitivity, and accurate results. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Further, the present invention also provides a method for preparing the above-mentioned immunofluorescence chromatography kit for quantitative detection of SAA, CRP, and PCT, comprising the following steps:
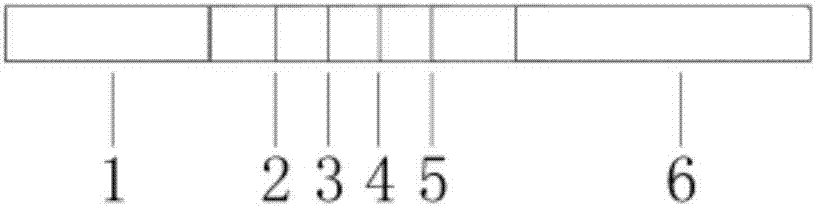
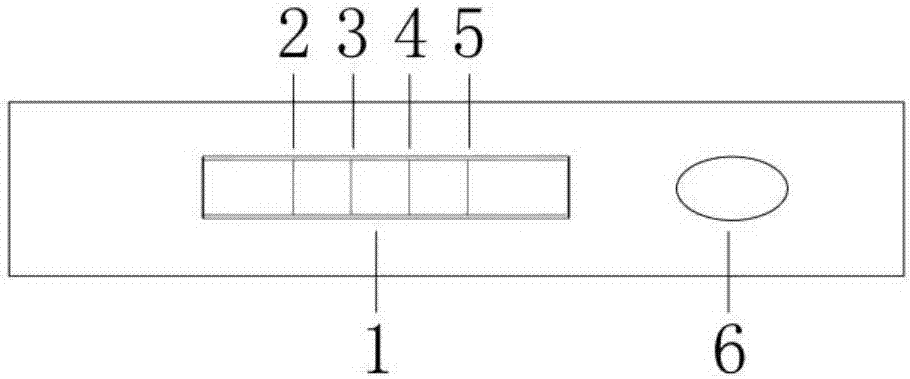
[0038] 1) Preparation of fluorescent immunochromatography test strips: paste nitrocellulose membrane on the backing of the test paper, affix an absorbent pad on one end of the nitrocellulose membrane, affix a sample pad on the other end, and absorb it near the nitrocellulose membrane. One end of the pad is sequentially marked with goat anti-rabbit IgG polyclonal antibody quality control line, SAA monoclonal antibody detection line, CRP monoclonal antibody detection line and PCT monoclonal antibody detection line to prepare a test paper board, and then use a strip cutter to cut the test paper The plate is cut longitudinally into 4mm wide test strips;
[0039] 2) Preparation of the fluorescent substance application solution: respectively couple the rabbit IgG polyclo...
Embodiment 1
[0045] This embodiment provides an immunofluorescence chromatography kit for the quantitative detection of SAA, CRP, and PCT and a preparation method thereof.
[0046] In this embodiment, the fluorescent substance is a time-resolved fluorescent microsphere with an excitation wavelength of 360 nm and an emission wavelength of 615 nm, which is a fluorescent dye polystyrene microsphere coated with rare earth europium element.
[0047] The preparation method of the immunofluorescence chromatography kit for the quantitative detection of SAA, CRP, PCT of this embodiment comprises the following steps:
[0048] (1) Activation of time-resolved fluorescent microspheres: First, take 1 mL of fluorescent microspheres and add 4 ml of 0.05 mol / L, pH 6.0 MES solution, and ultrasonically disperse them; weigh 5 mg of N-hydroxysuccinimide (NHS) and add into the microsphere suspension, then shake well to dissolve; then weigh 5 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC...
Embodiment 2
[0057] This embodiment provides an immunofluorescence chromatography kit for the quantitative detection of SAA, CRP, and PCT and a preparation method thereof.
[0058] The structure of the test strip in this example is the same as that in Example 1, the difference is that the fluorescent substance in Example 2 is a fluorescent microsphere with an excitation wavelength of 470nm-490mm and an emission wavelength of 525-560nm. Dyed polystyrene microspheres.
[0059] The preparation method of the immunofluorescence chromatography kit for the quantitative detection of SAA, CRP, PCT of this embodiment comprises the following steps:
[0060] (1) Activation of fluorescent microspheres: first take 1mL of fluorescent microspheres and use ultrasonic waves to disperse them by ultrasonic waves. The treatment time is 30s with 100W ultrasonic waves; min, centrifuged for 20 min, poured off the supernatant, and added 1 mL of 0.1 mol / L, pH 4.7 MES buffer. Weigh 3 mg of N-hydroxysuccinimide (NH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com