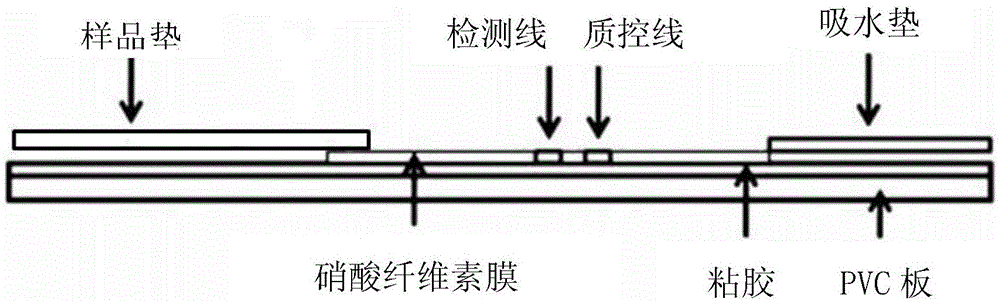
Kit for time resolution fluorescent quantitative detection on PCT
A technology of time-resolved fluorescence and quantitative detection, which is applied in the field of immunoassay, can solve the problems of decreased sensitivity and achieve the effects of stable labeled products, fast and easy detection, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 Time-resolved fluorescence quantitative detection kit preparation of PCT
[0047] (1) Preparation of fluorescent microsphere antibody complex
[0048] ① Take 0.5mL rare earth fluorescent microspheres (lanthanides) (purchased from Shanghai Weikeli, product number: DF020EU), centrifuge to get the precipitate, add MES buffer of pH 6.0 to wash twice. MES buffer formula: Weigh 9.76g MES (2-(N-morpholine) ethanesulfonic acid), 29.22g NaCl, 5ml 10% Brij35, dissolve in 1L distilled water, adjust pH=6.0 with 3mol / L NaOH.
[0049] ②Activation: 114 μL of 50 mM carbodiimide (EDAC) and 114 μL of 50 mM hydroxysulfosuccinimide (Sulfo-NHS) were added to the washed microspheres respectively, and shaken at room temperature for 1 h.
[0050] ③Labeled antibody: Centrifuge the microspheres to get the precipitate, wash twice with PB buffer.
[0051] ④ Add an appropriate amount of PCT monoclonal antibody A and shake at room temperature for 2 hours to make the carboxyl group and...
Embodiment 2
[0074] Embodiment 2 uses kit of the present invention to detect PCT standard substance
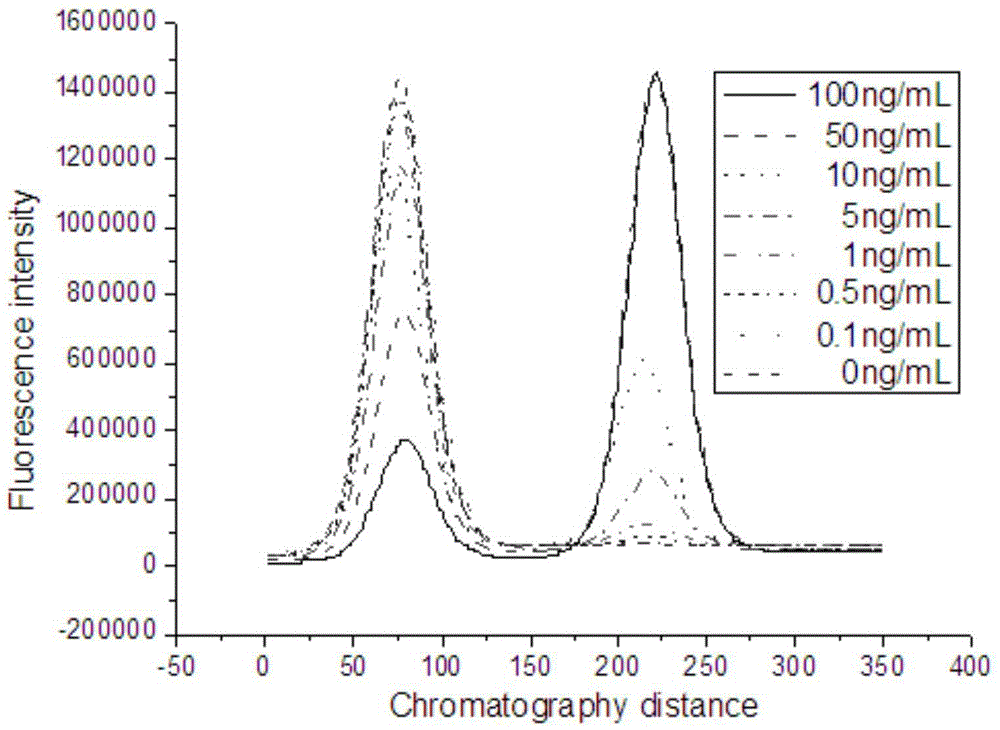
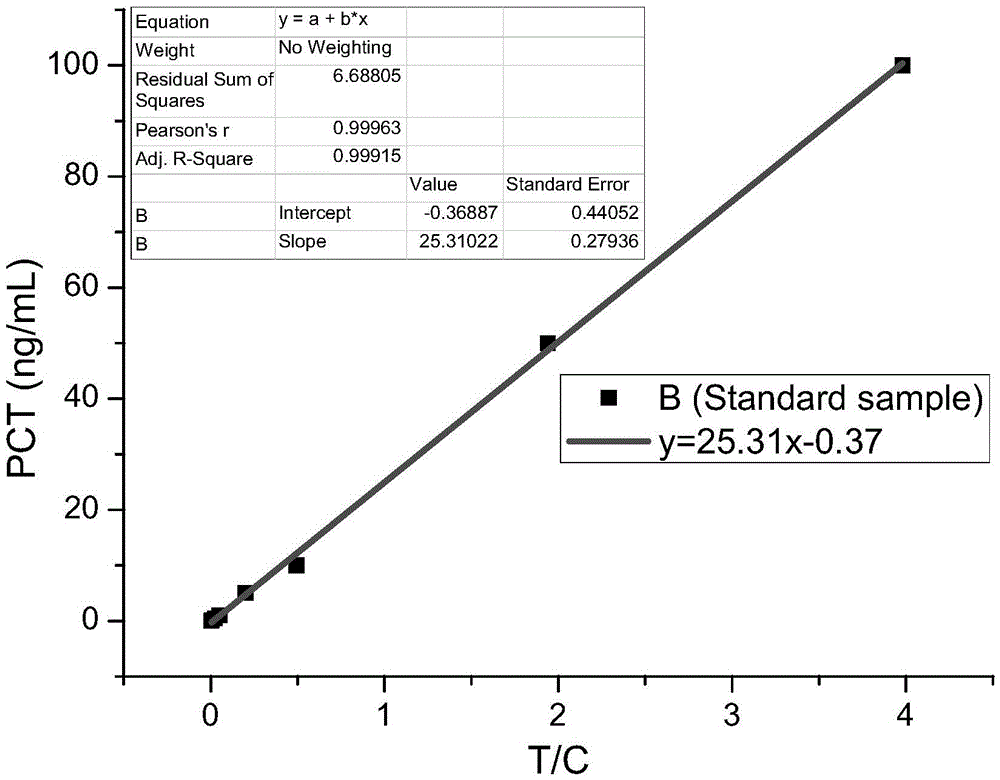
[0075] Serially dilute the PCT standard (our company), and use the sample diluent to dilute the standard 10 times to make samples of 100000ng / L, 10000ng / L, 1000ng / L, 100ng / L, and 10ng / L. Install the tip containing the freeze-dried fluorescent microsphere complex on the pipette to take 75 μL of sample solution, add it to 300 μL of sample buffer (0.25% casein, 40 mM EDTA, PB), mix well, take 75 μL and add it to In the sample hole of the test strip card. Let it stand at room temperature for 20 minutes, and put the test paper card into the fluorescence scanner for reading. Divide the fluorescence value of the detection line by the fluorescence value of the quality control line to measure the value. Each sample concentration was measured three times, and after taking the average value, the measured value was plotted against the sample concentration. The peak shape of the detection line and q...
Embodiment 3
[0076] The detection of PCT in the clinical blood sample of embodiment 3
[0077] The positive and negative samples of PCT in the hospital were collected, tested with this kit (method as above) and EISA, and the detection rate was calculated respectively. The results are shown in Table 1. The detection rate of the kit for time-resolved fluorescence quantitative detection of PCT of the present invention is significantly higher than that of the ELISA method.
[0078] Table 1. Detection of PCT in clinical blood samples
[0079]
[0080] At the same time, the accuracy correlation experiment was carried out on the blood samples, and the detection results of the ELISA detection results were plotted with the detection results of the time-resolved fluorescence quantitative detection kit for PCT, as shown in Figure 4 As shown, the distribution of points is mainly distributed near the 45-degree straight line, and the correlation curve is obtained by fitting, and the correlat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com