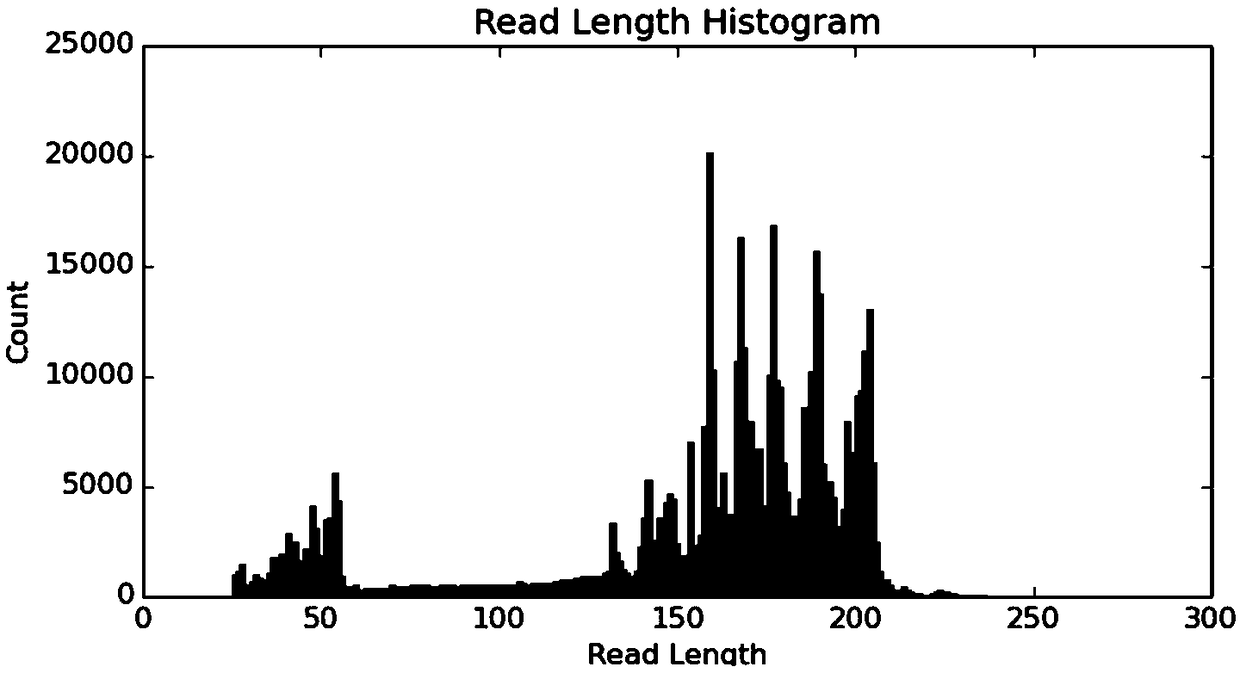
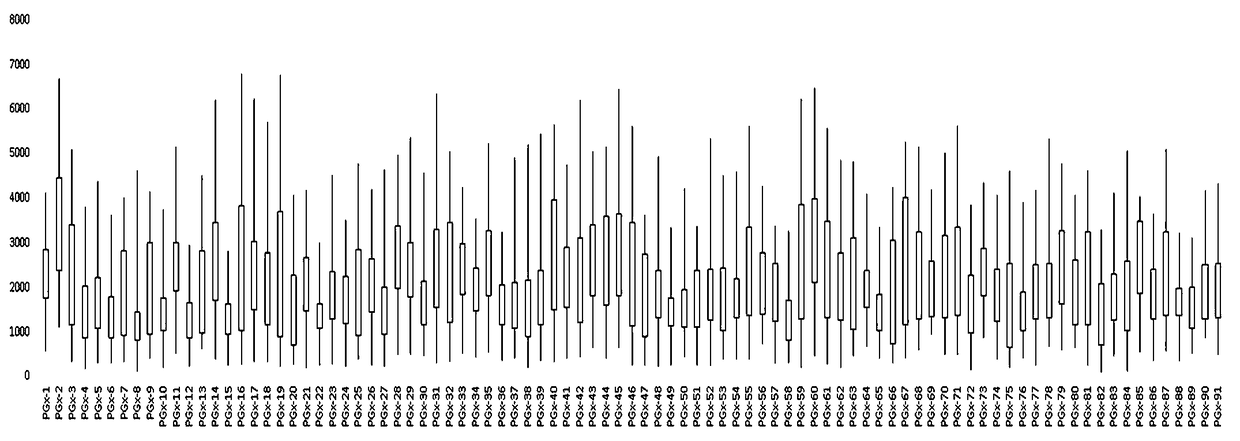
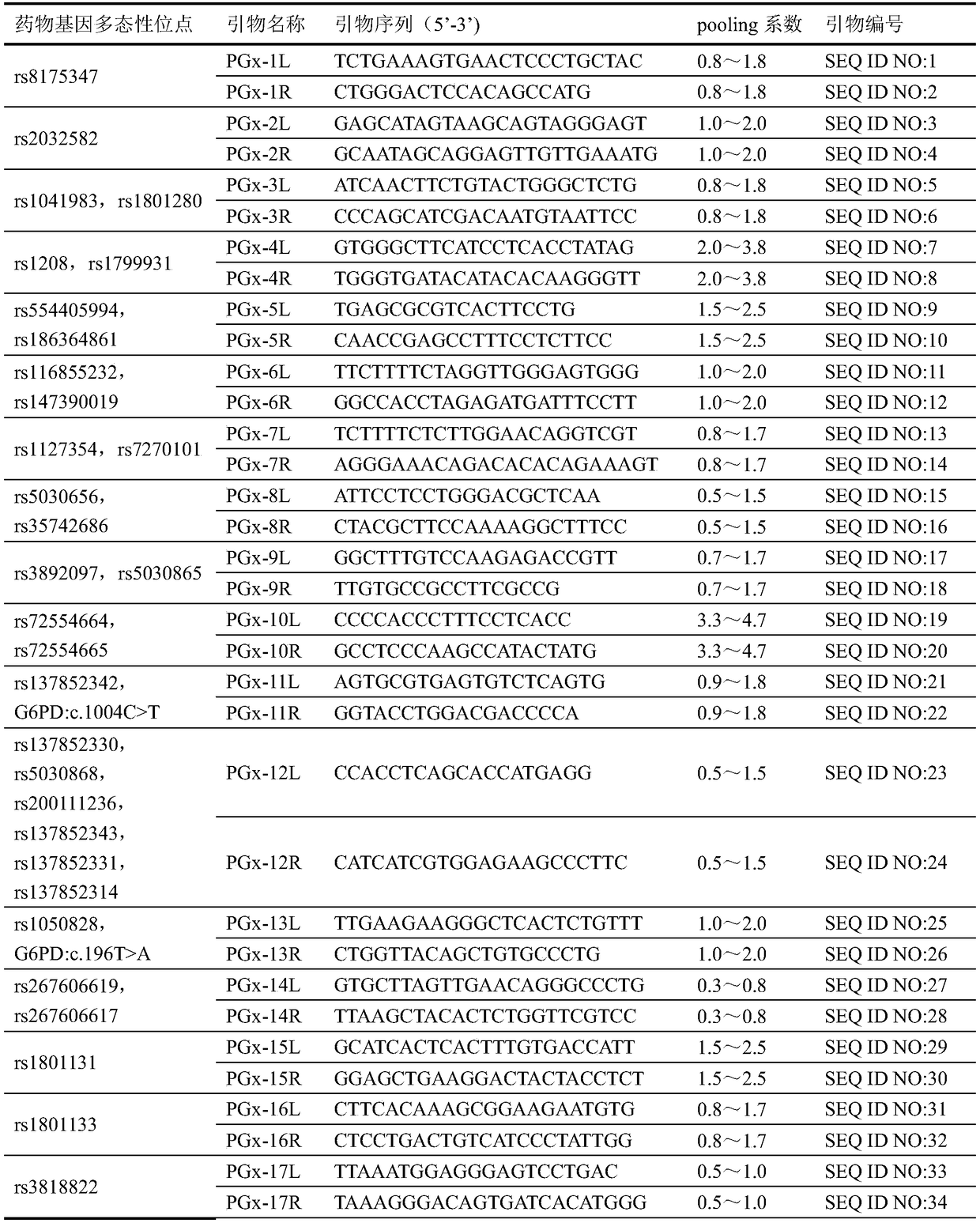
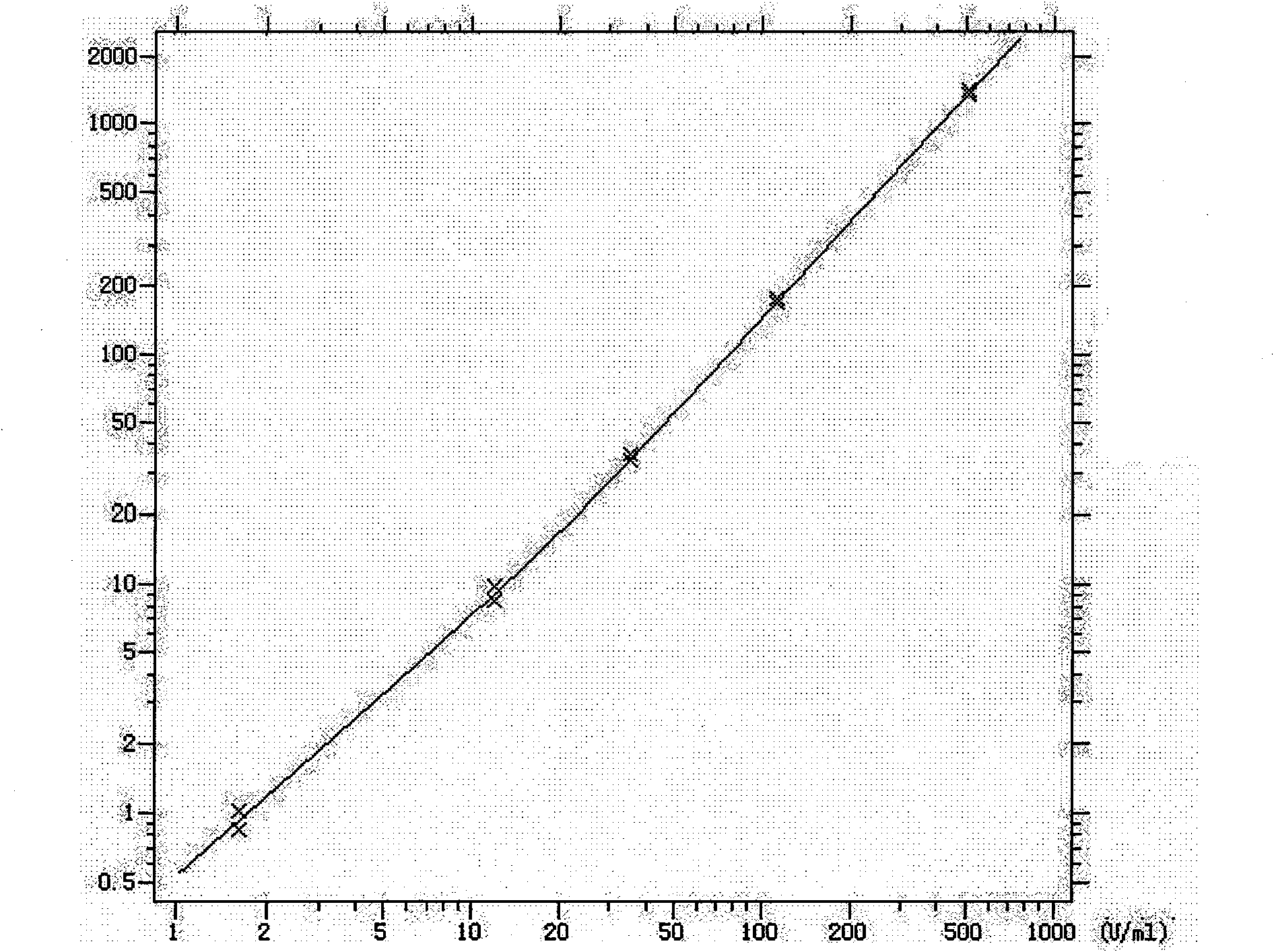
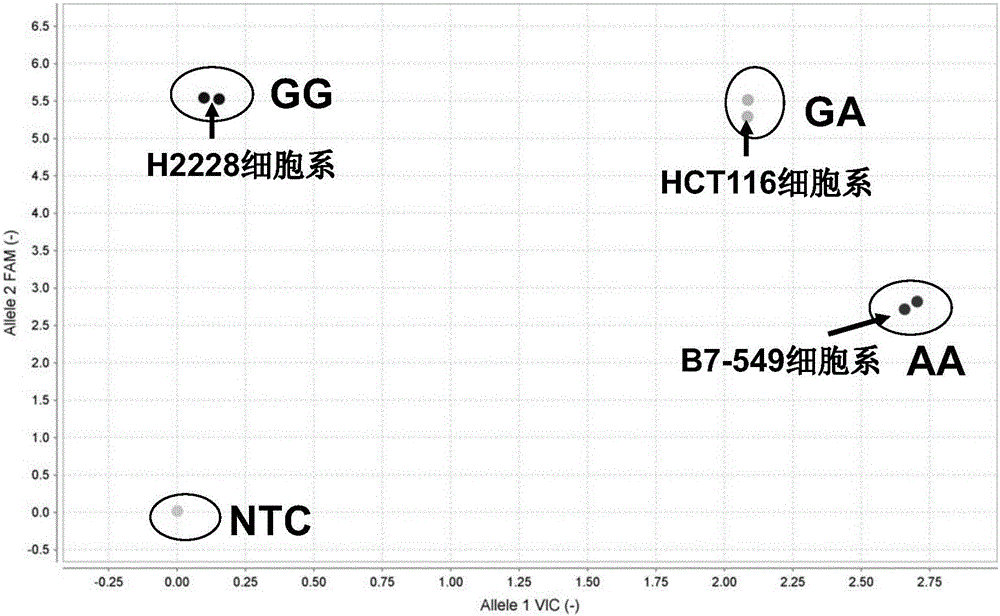
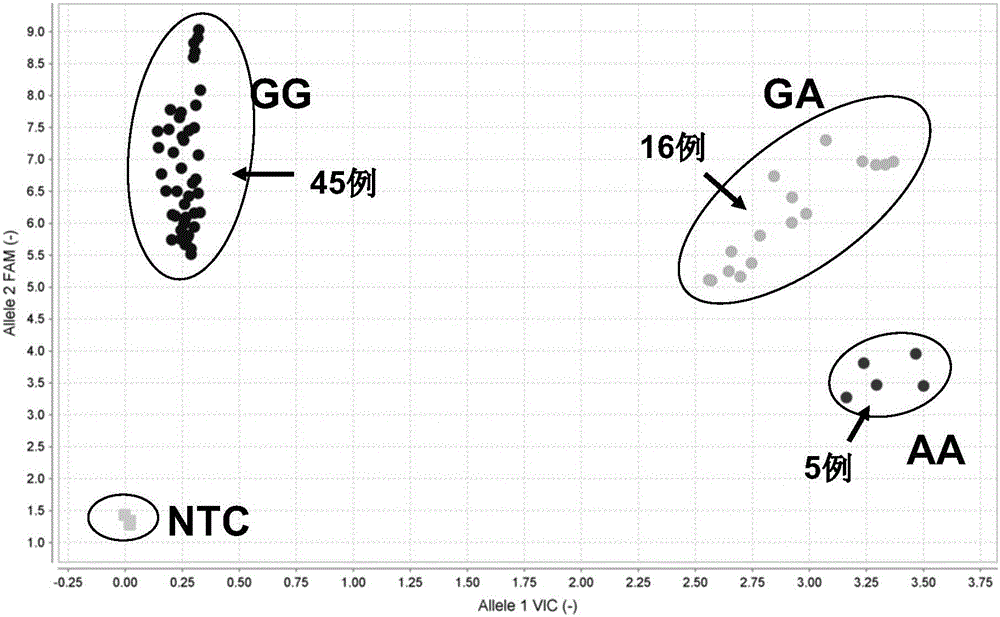
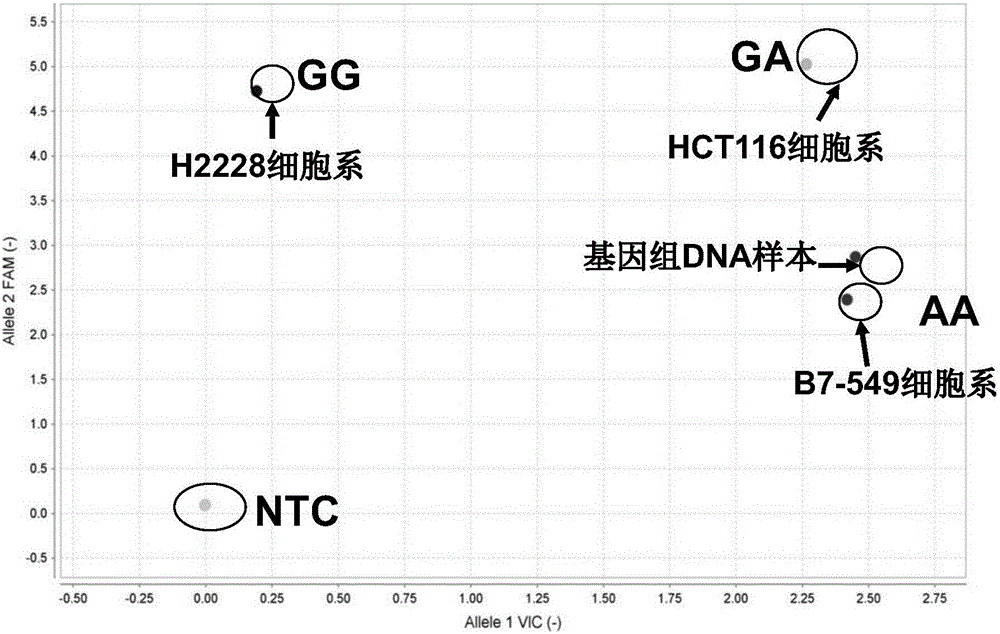
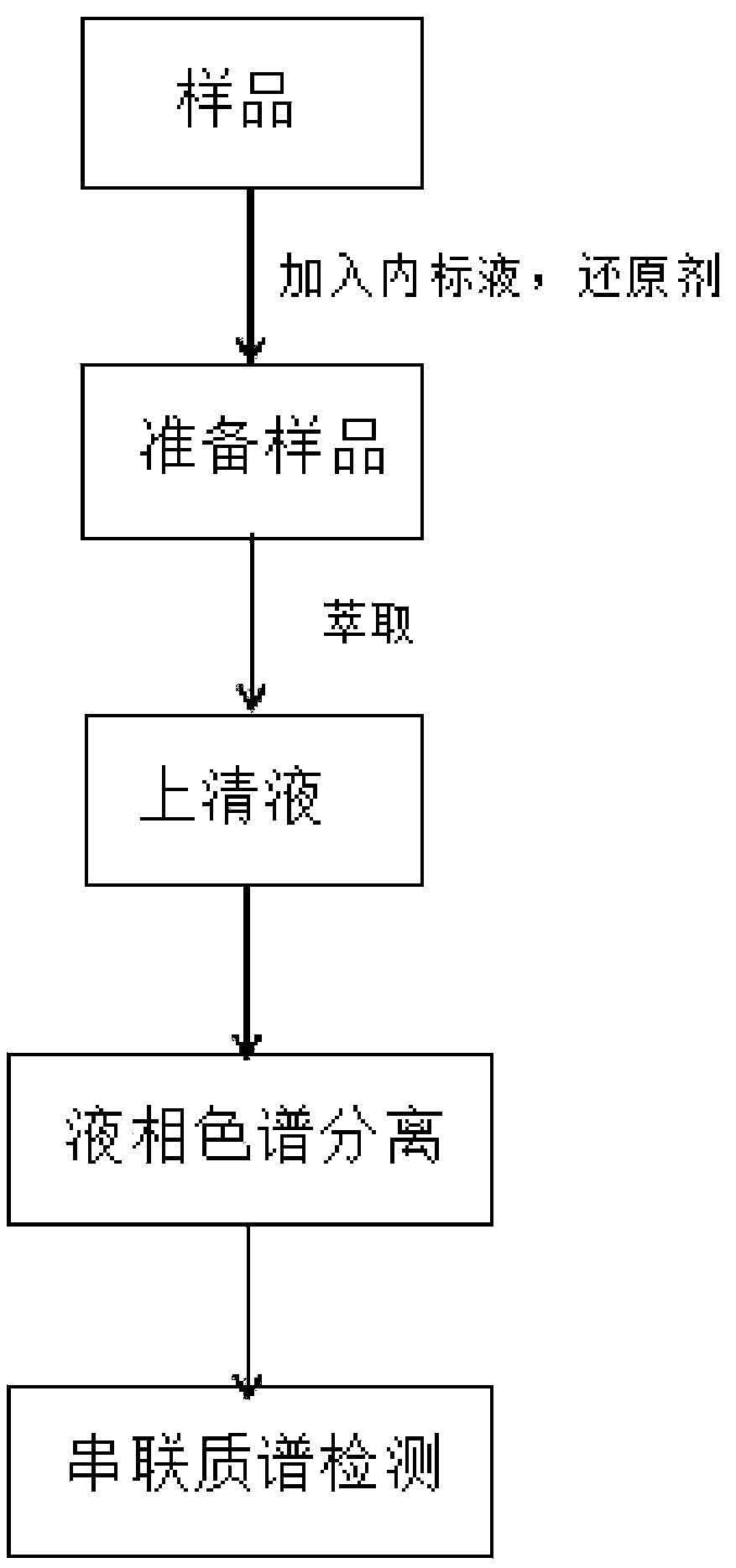
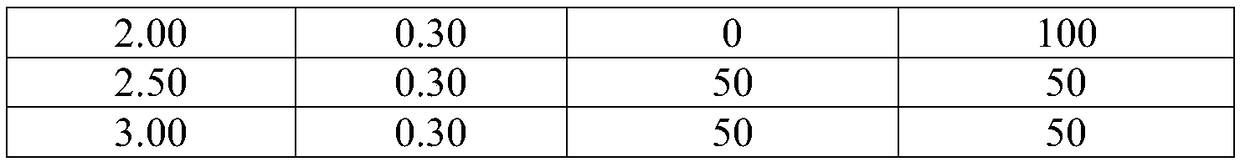
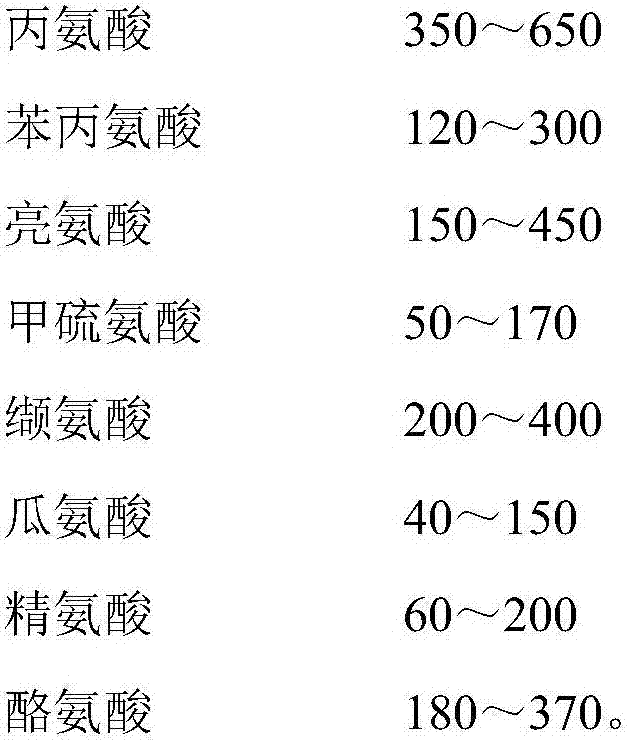
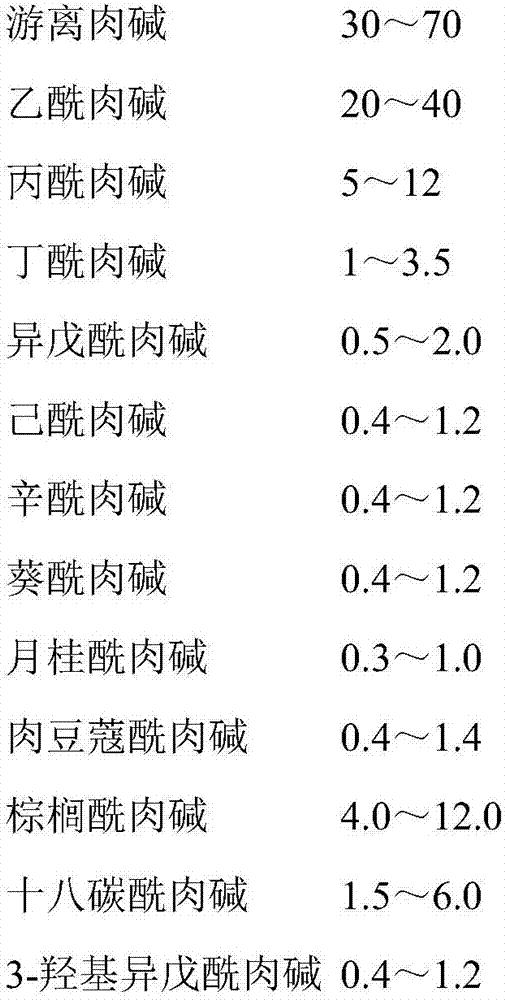
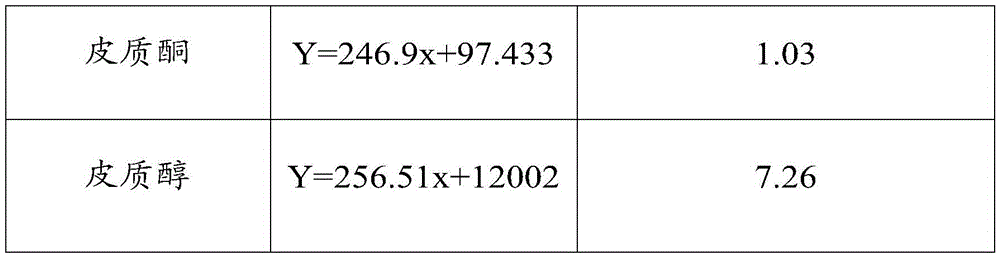Patents
Literature
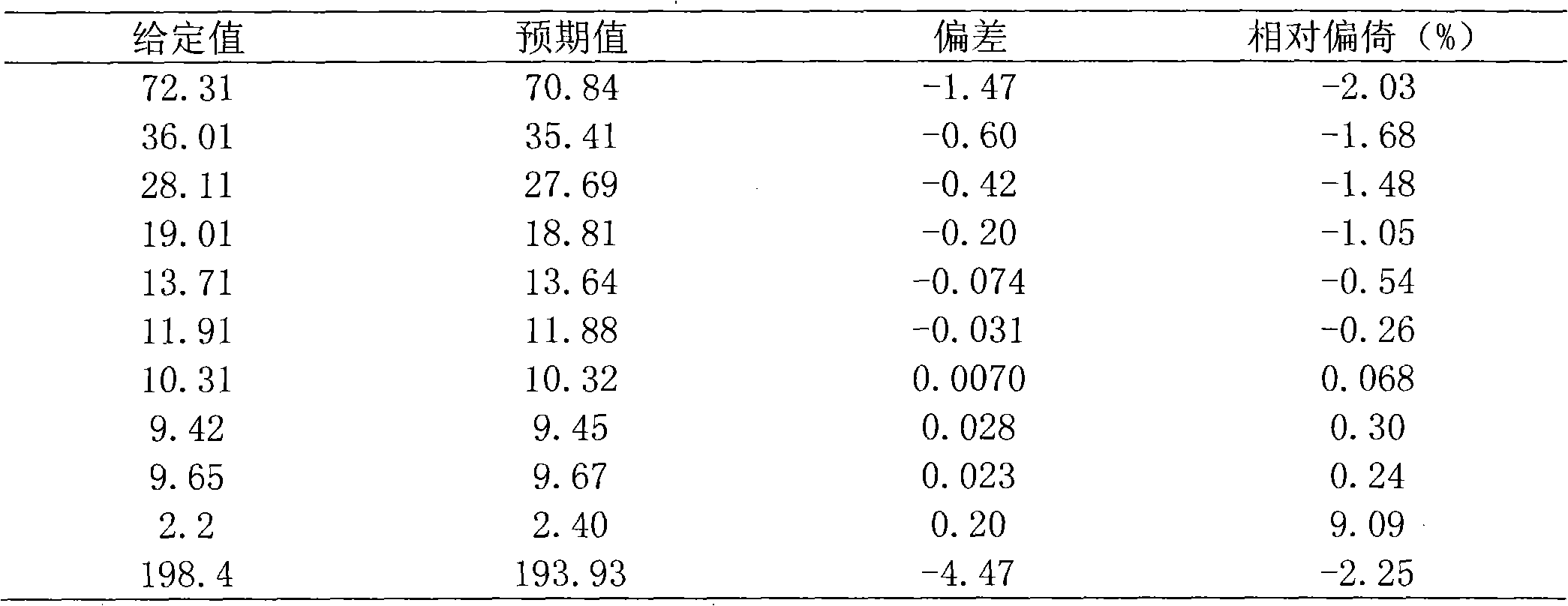
146 results about "Dried blood spot" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dried blood spot testing (DBS) is a form of biosampling where blood samples are blotted and dried on filter paper. The dried samples can easily be shipped to an analytical laboratory and analysed using various methods such as DNA amplification or HPLC.
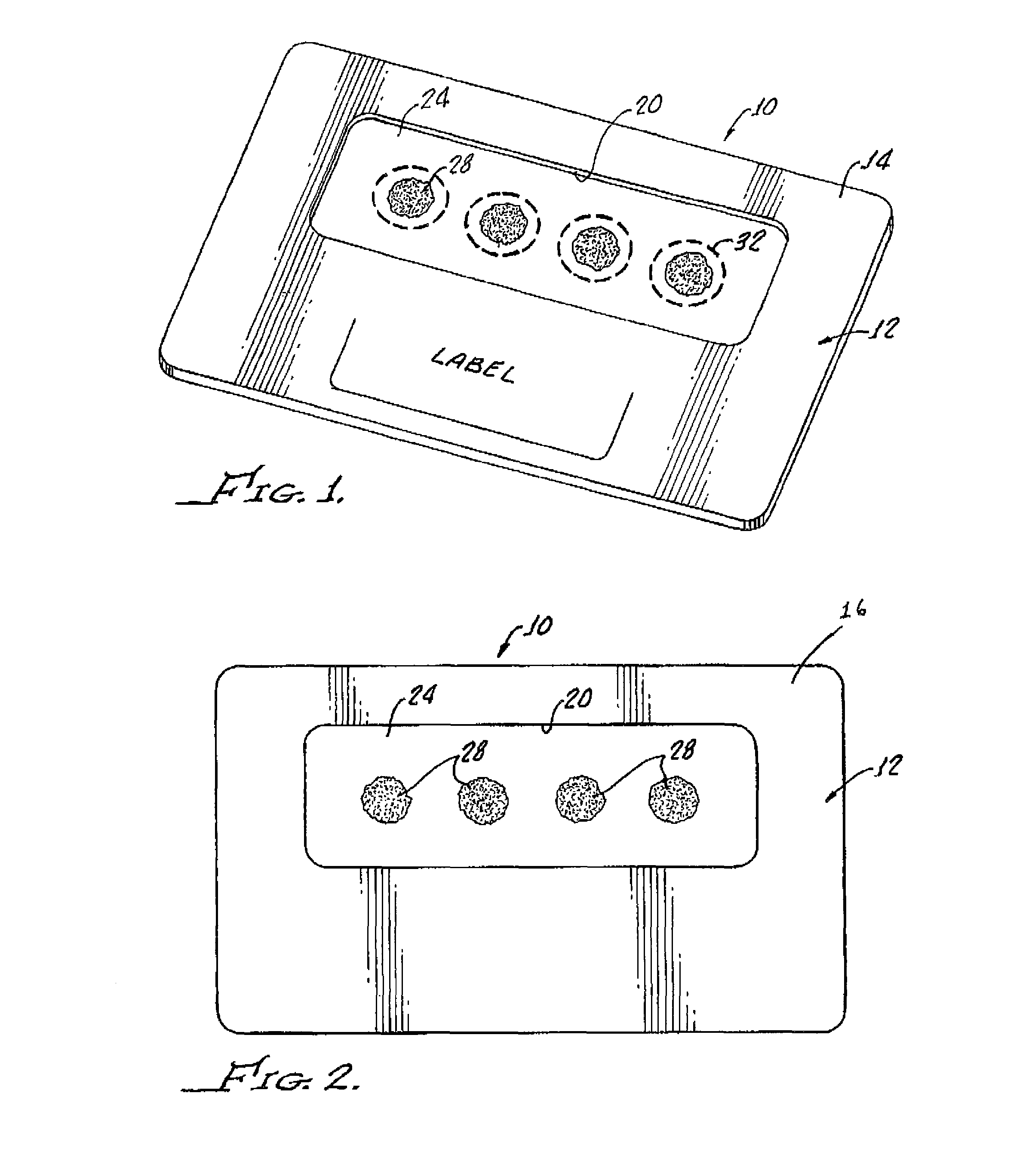
Systems and methods for collection and/or manipulation of blood spots or other bodily fluids
InactiveUS20120277629A1Easy to operateReduction and elimination of painHaemofiltrationMedical devicesFilter paperBody fluid
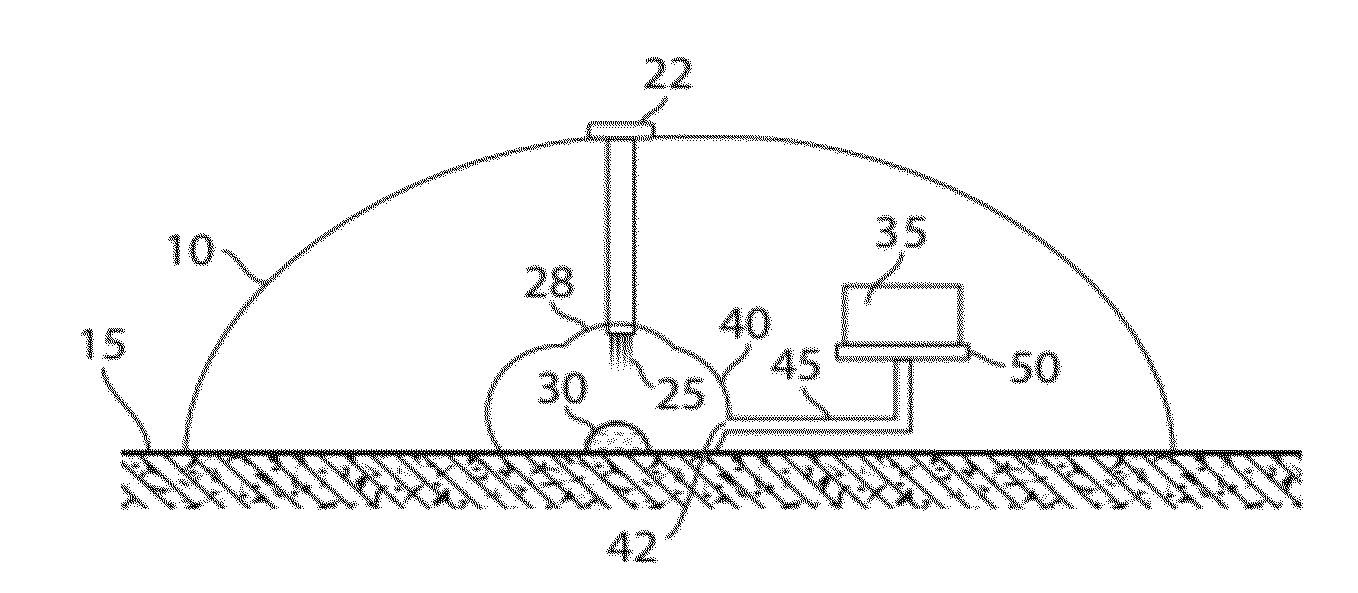
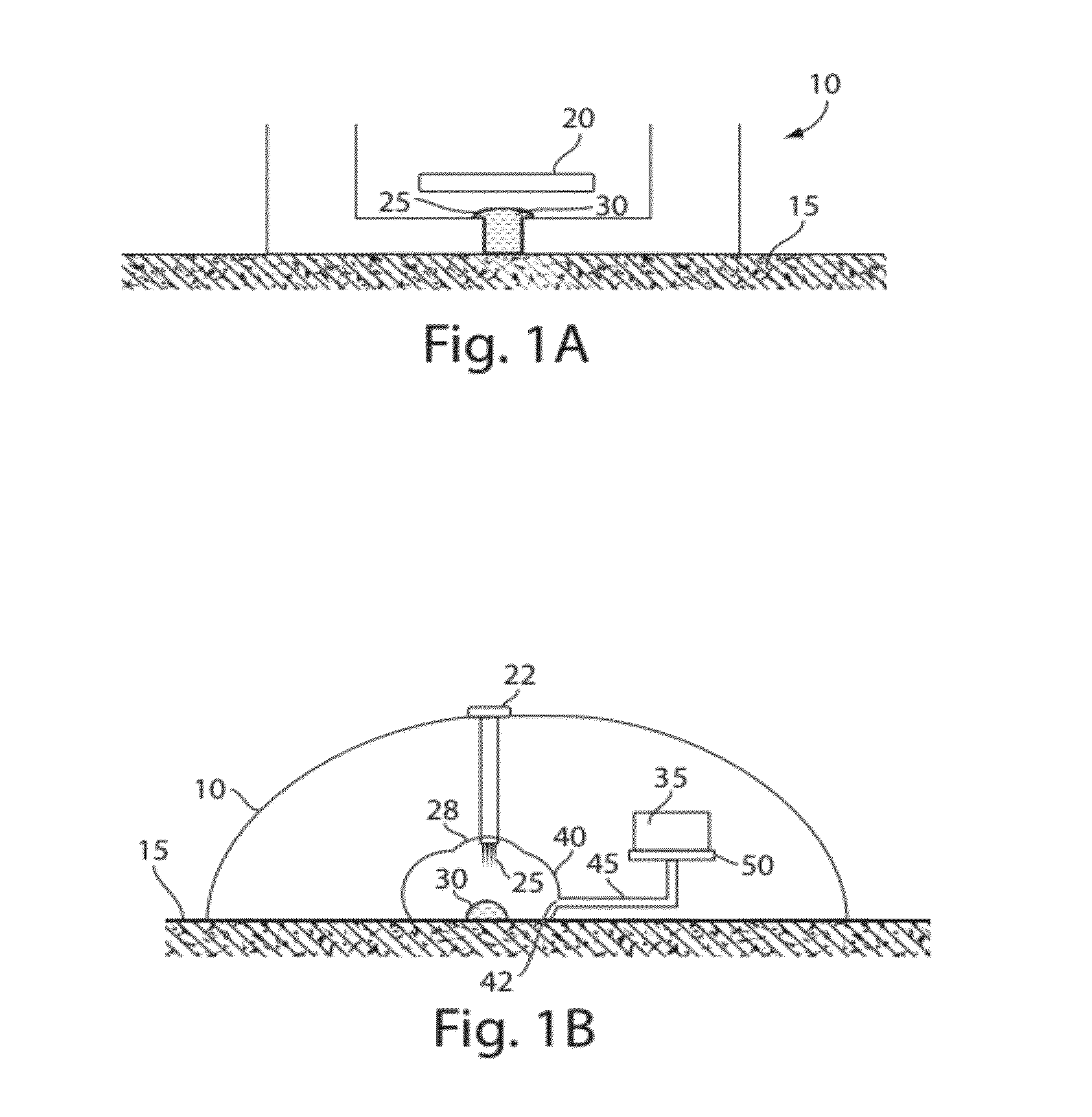
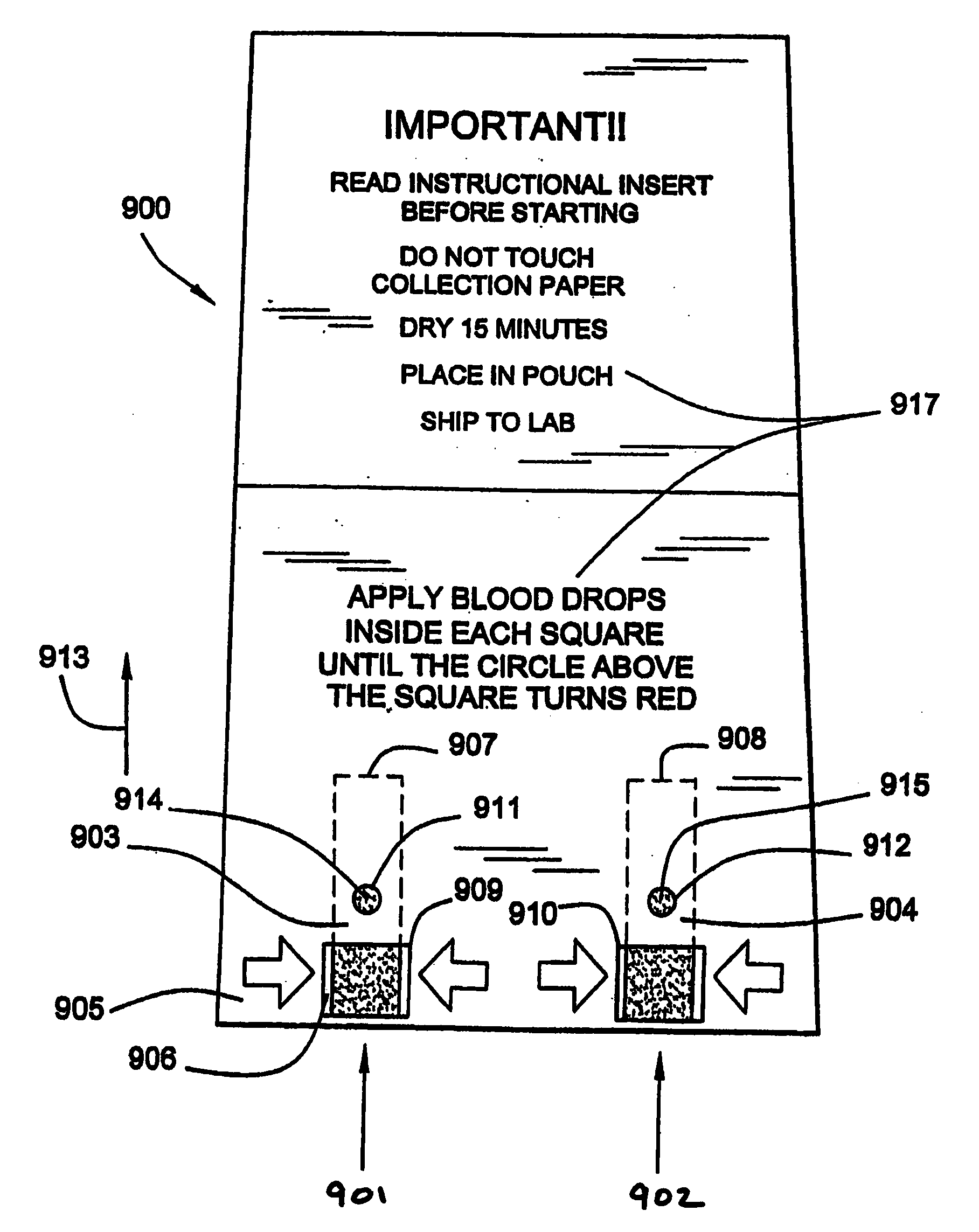
The present invention generally relates to systems and methods for receiving blood (or other bodily fluids) from a subject, e.g., from or beneath the skin of a subject. In some cases, the blood (or other bodily fluids) may be deposited on a membrane or other substrate. For example, blood may be absorbed in a substrate, and dried in some cases to produce a dried blood spot. In one aspect, the present invention is generally directed to devices and methods for receiving blood from a subject, e.g., from the skin, using devices including a substance transfer component (which may contain, for example, one or more microneedles), and directing the blood on a substrate, e.g., for absorbing blood. The substrate, in some embodiments, may comprise filter paper or cotton-based paper. After absorption of some blood onto the substrate, the substrate may be removed from the device and shipped or analyzed.
Owner:SEVENTH SENSE BIOSYST
Analyte recovery from dried blood spots
InactiveUS20110136251A1Minimize the numberQuantity minimizationWithdrawing sample devicesPreparing sample for investigationAnalyteSorbent
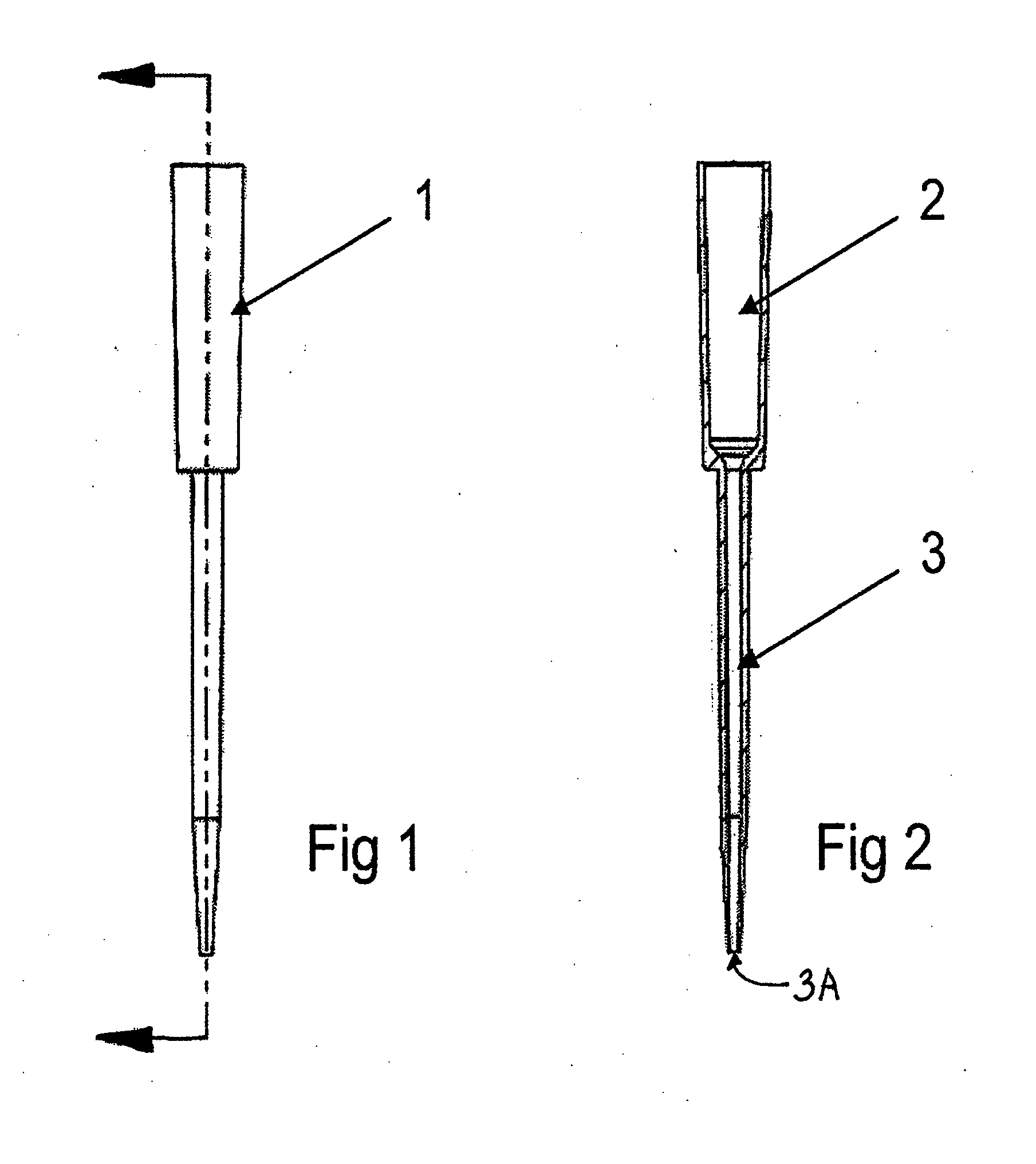
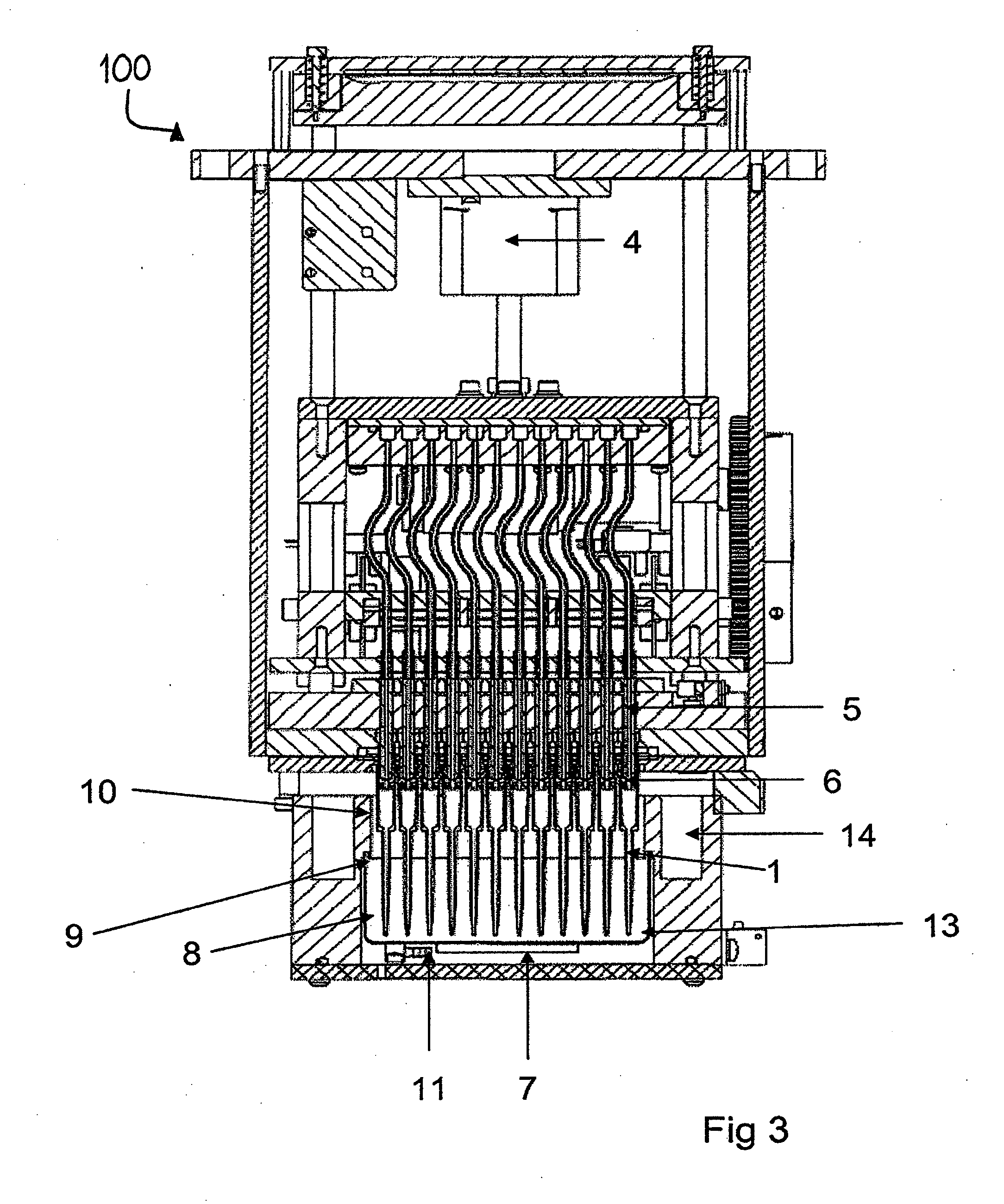
Pipette tips, pipetting system and methods for eluting an analyte from a solid phase filtration media that resides within an upper portion of the pipette tips. Each pipette tip includes an upper barrel containing and retaining the solid phase filtration media and a lower cannula. The solid phase filtration media resides between the upper barrel and the lower cannula. An eluting solvent is provided into the pipette tips for eluting the analyte from the solid phase filtration media by soaking, ultrasonic agitation or agitation by mixing. A sorbent material may reside within the cannula portion of each pipette tip for filtering or treating the eluted analyte containing solution prior to it being dispensed from the pipette tip into a microplate for subsequent analytical processing.
Owner:ASTLE THOMAS W
Method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine
InactiveCN108333268AAvoid distortionRealize real-time monitoringComponent separationThree levelChromatographic separation
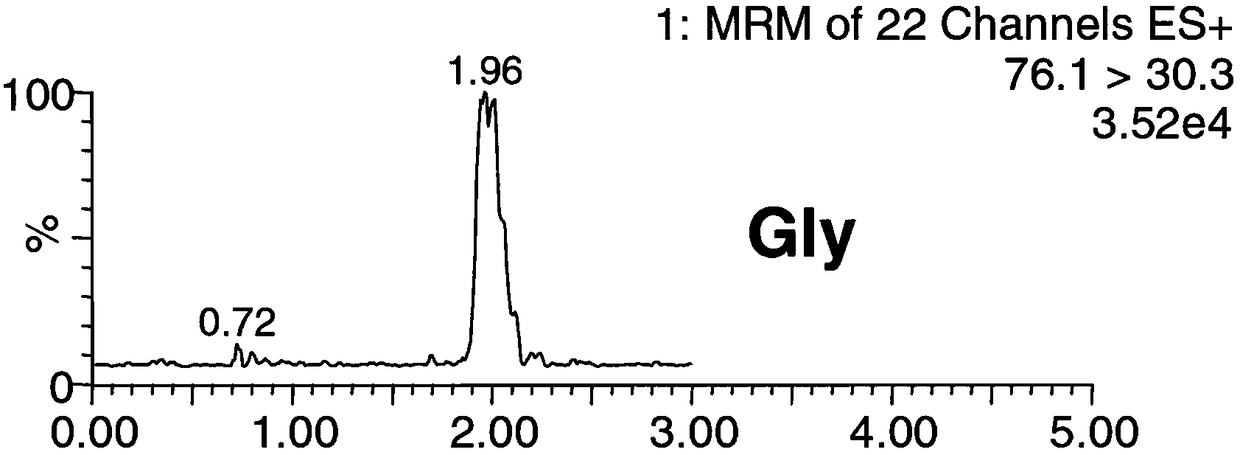
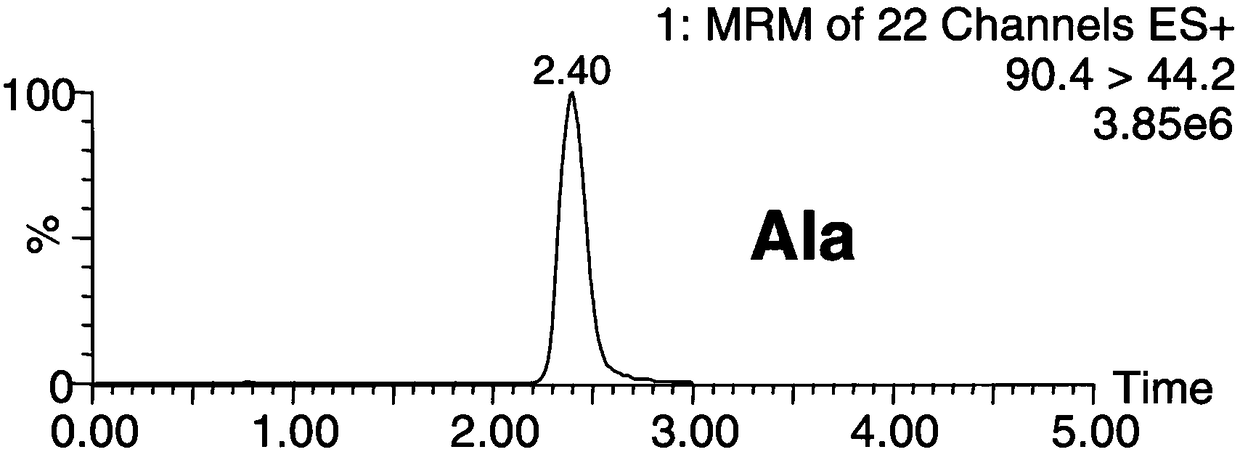
The invention discloses a method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine. The method comprises the following steps of firstly, pretreating a biological sample by a simple liquid and liquid extracting method; performing chromatographic separation and mass spectrometric detection; using the relative retention time and qualitative ions as the qualitative basis for each type of amino acid, and using a standard product to manufacture a standard curve for quantifying. The method has the advantages that the accuracy and validity of the method shall be studied by three levels of quality control products, so as to avoid the distortion of detection results; the forty types of non-derived amino acids in one sample can be simultaneously detected bya HPLC-MS / MS (high performance liquid chromatography-mass spectrograph) technique for the first time; the operation is simple, convenient and rapid, the flux is high, and the cost is low; the level of amino acids in a human body can be effectively monitored in real time; the enough basis is provided for the clinical disease diagnosis; the method is suitable for being clinically popularized and applied.
Owner:JINAN YING SHENG BIOTECH
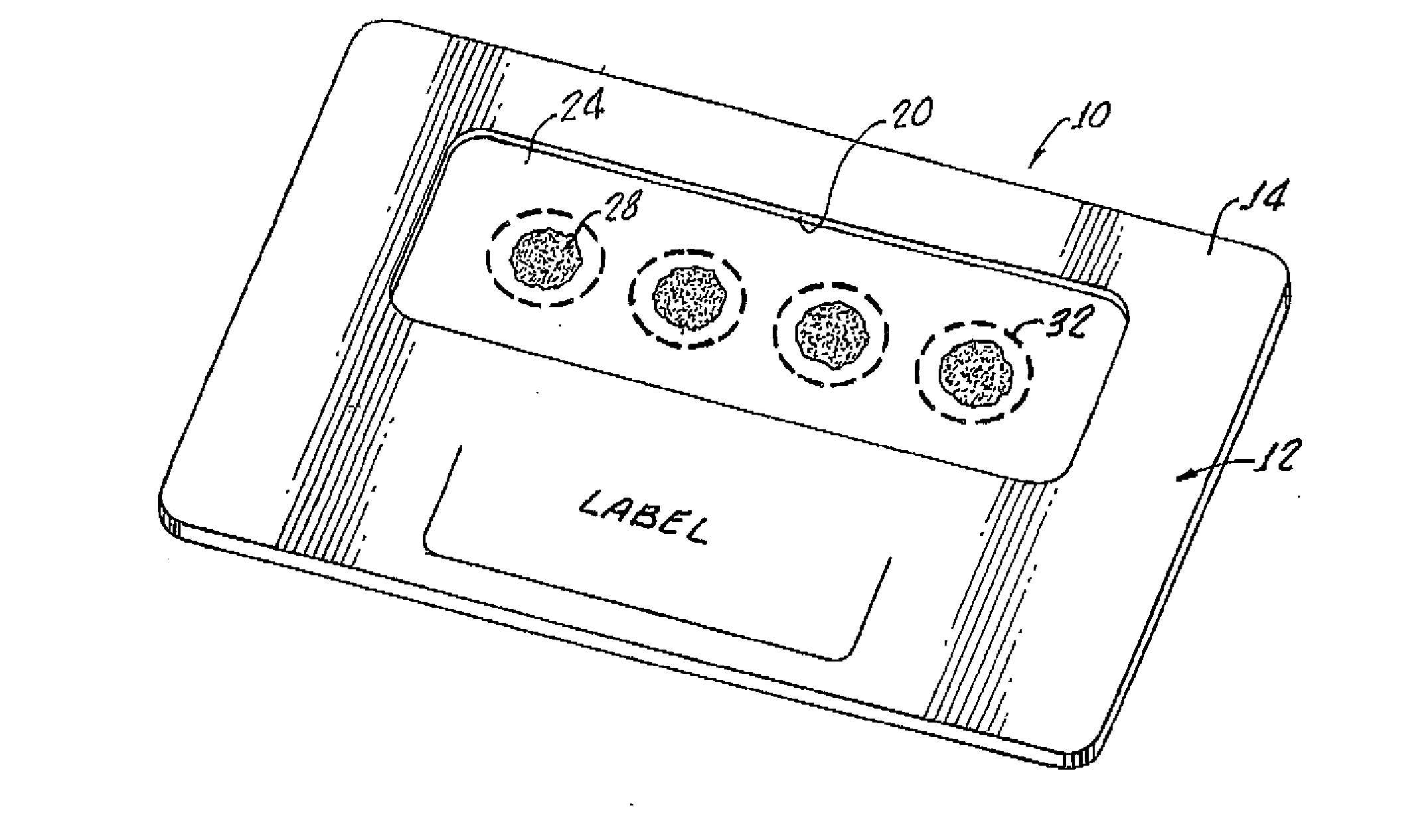
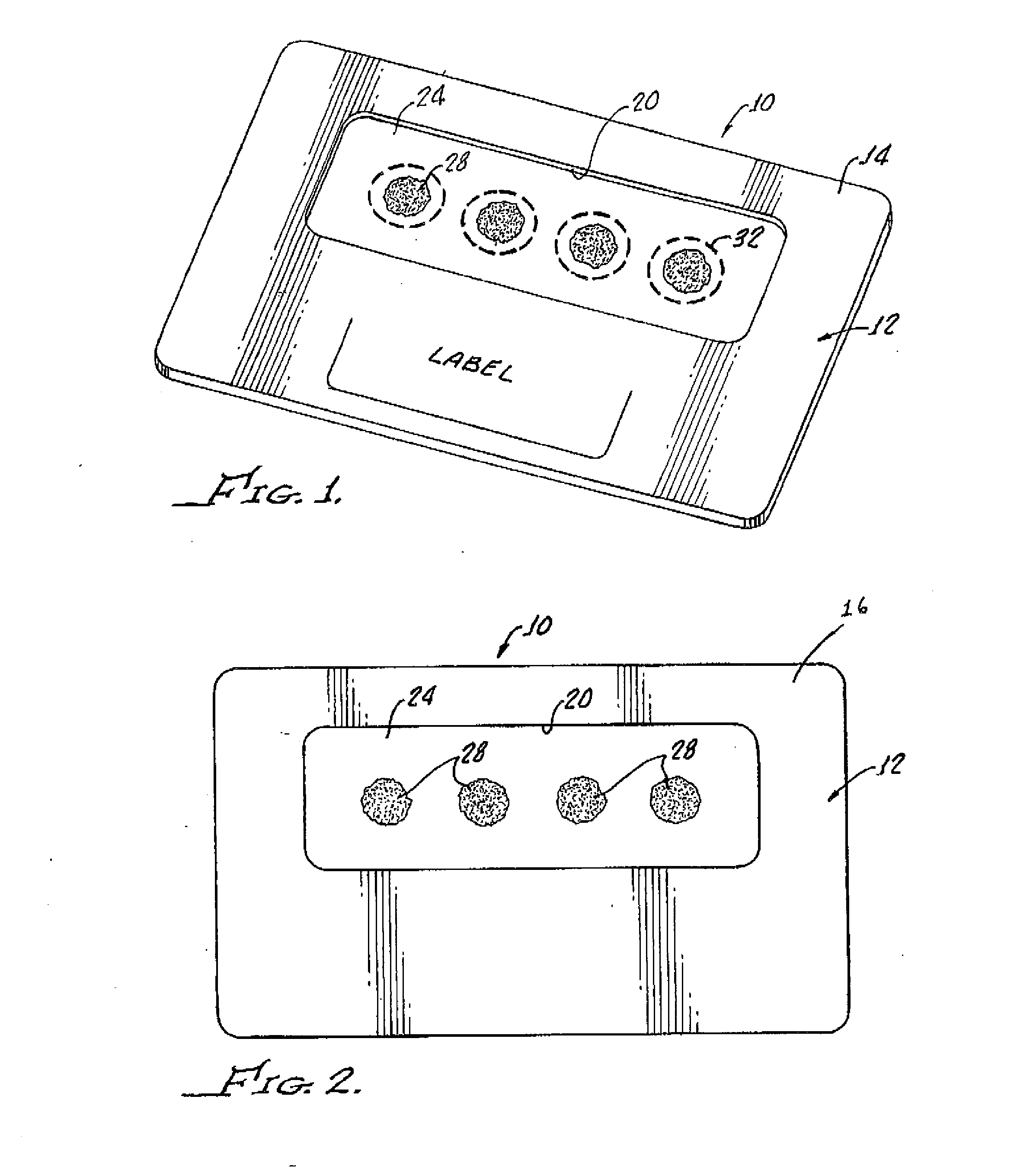
Dried blood spotting paper device and method
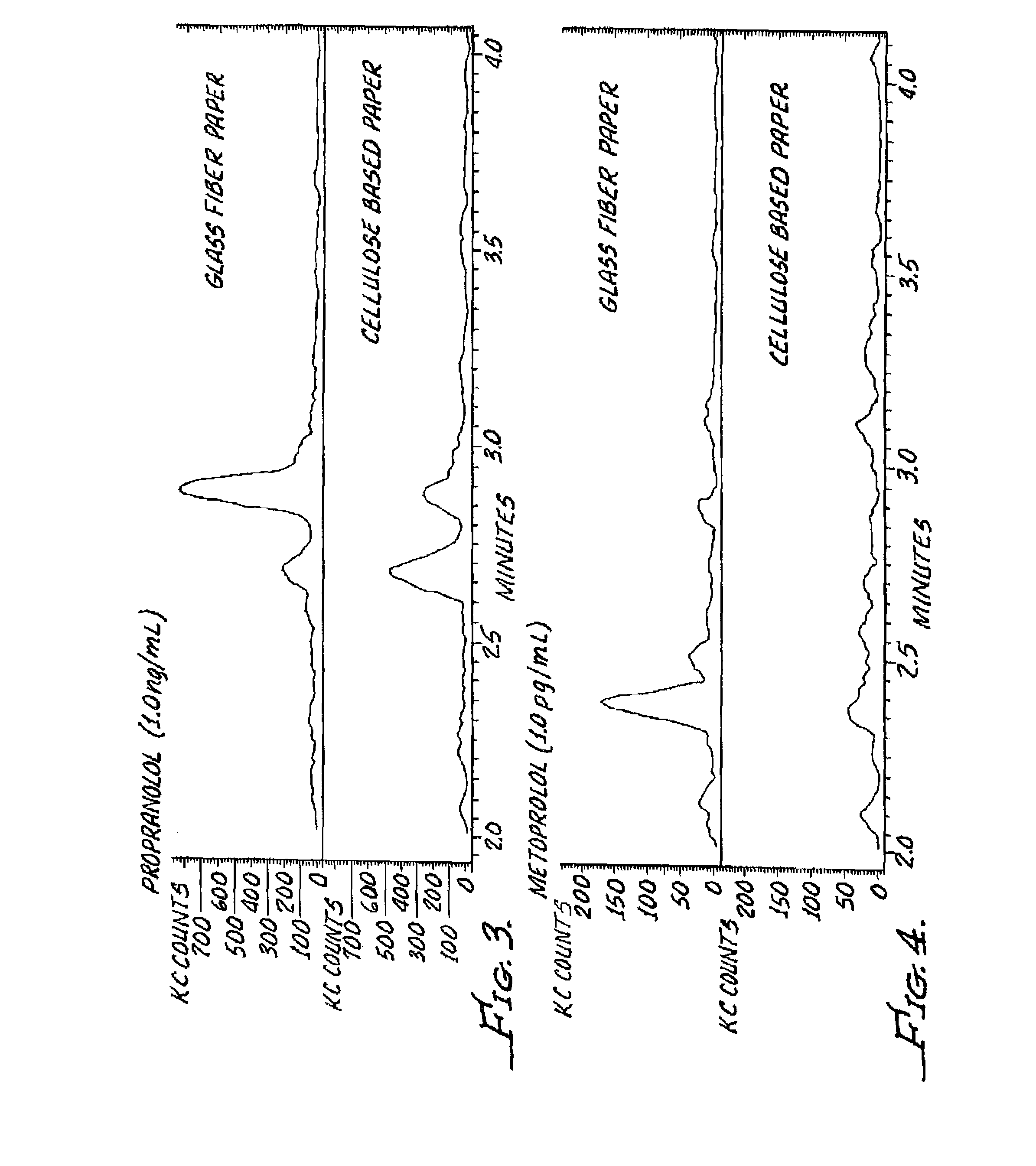
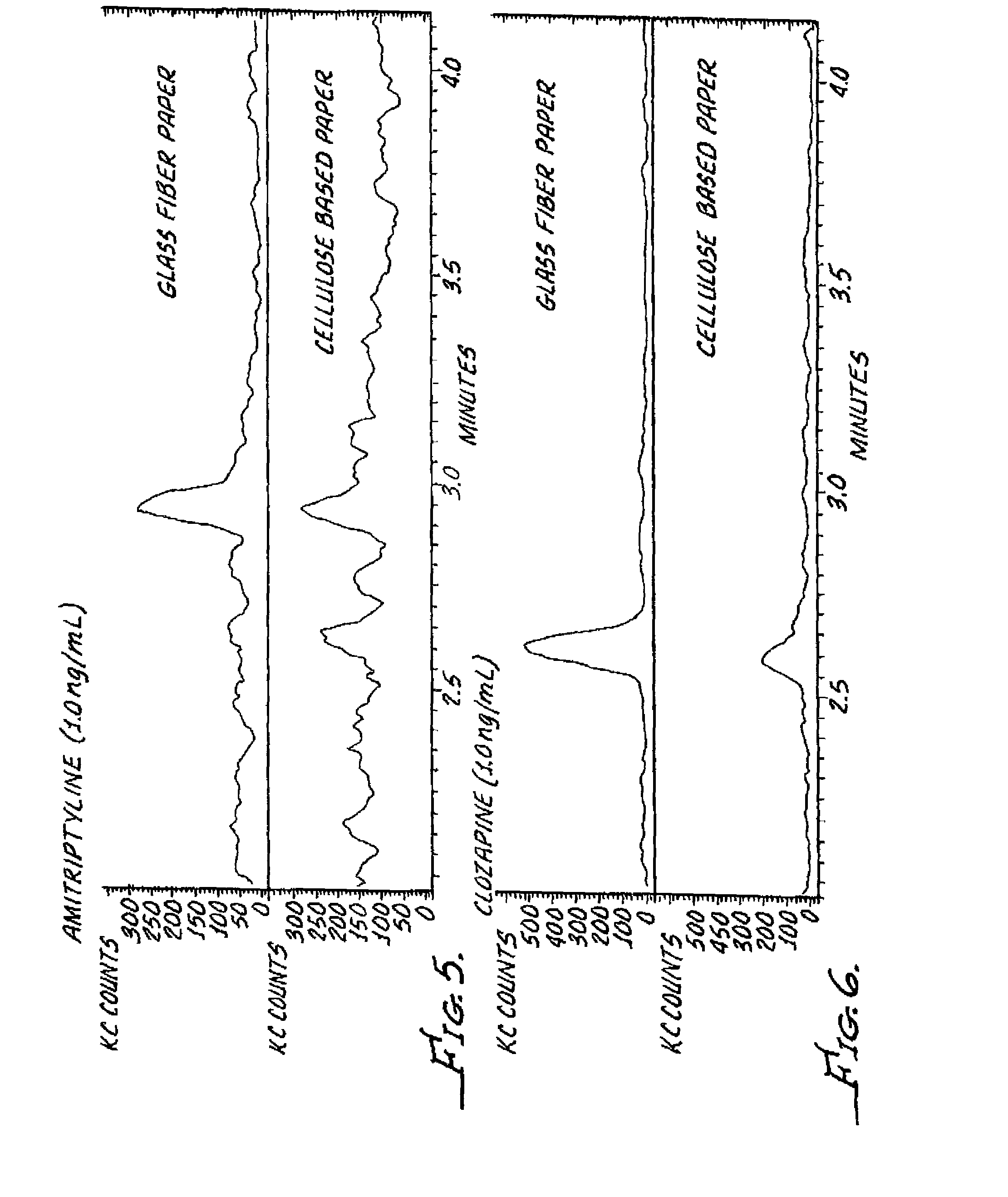
InactiveUS20120045792A1Bioreactor/fermenter combinationsBiological substance pretreatmentsGlass fiberSpectroscopy
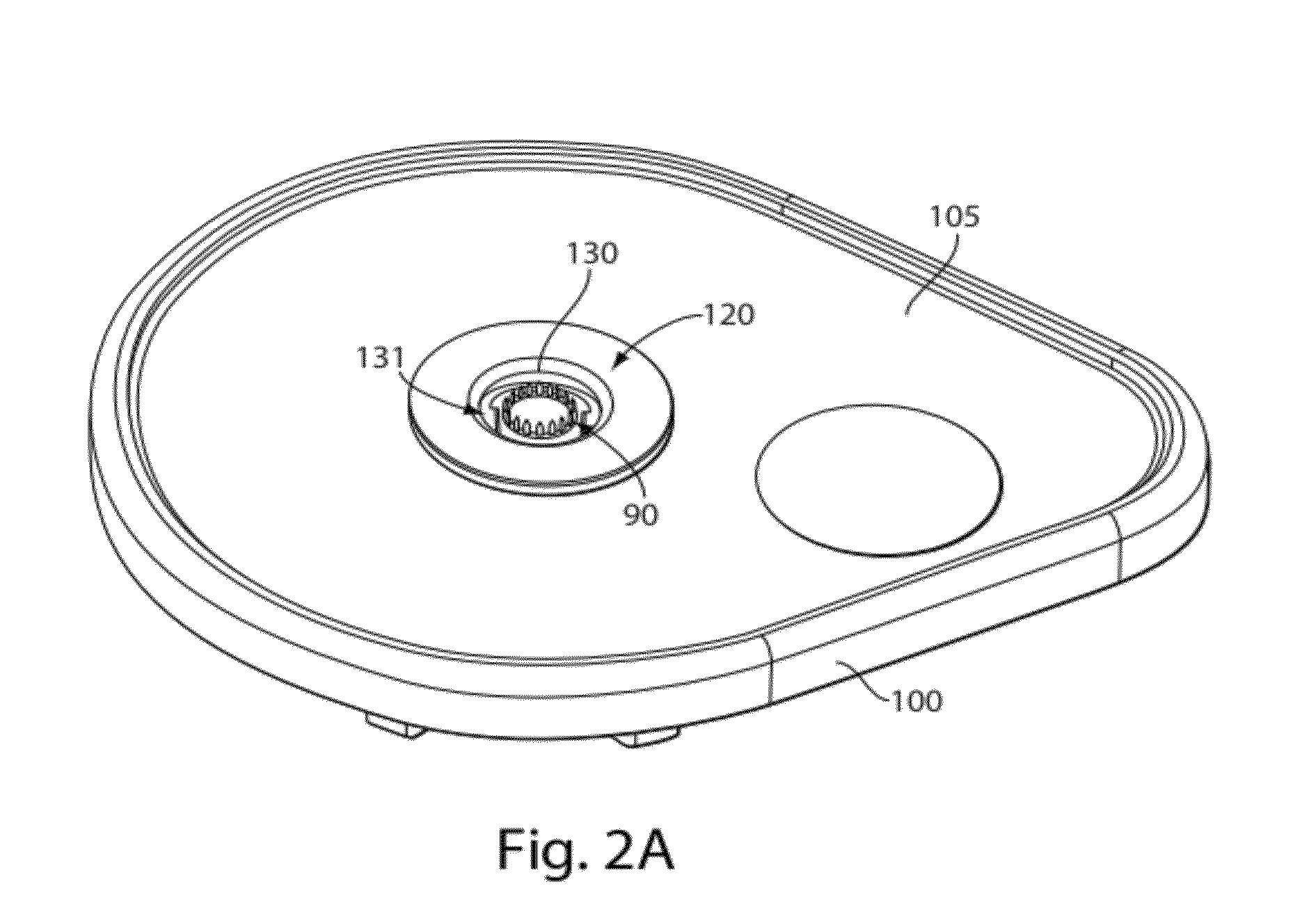
A dried blood-spotting device includes a carrier card having a window therethrough along with a punchable glass fiber paper disposed in the window for a uniform absorption of a blood droplet sample into a homogeneous circular sport. The glass fiber paper is configured for enabling improved analysis for small molecules by a liquid chemotography / mass spectroscopy of punched specimens of the sample absorbed glass fiber paper.
Owner:AGILENT TECH INC
Personalized medication gene detection kit and application
ActiveCN108707658AStrong specificityAvoid mutual interferenceMicrobiological testing/measurementLibrary creationMultiplex pcrsDried blood spot
The invention discloses a personalized medication gene detection kit and application. The personalized medication gene detection kit is suitable for multiple kinds of sample types including dried blood spots, whole blood and buccal swabs, information of all medicine gene polymorphic sites can be acquired at once, the sequencing effective rate is 50% or above, the sequencing cover degree is 100%, the average sequencing depth exceeds 3000 X, the library building success rate is larger than or equal to 98.5%, and the medicine gene polymorphic site detection accuracy is larger than or equal to 99.9%; a primer group is high in specificity and little in nonspecific amplification, interference among primers can be well overcome, under the situation of up to 186 primer sequences, one-tube multiplePCR reaction is achieved, moreover, amplicon is high in uniformity, and under the situation of large amplification area GC content difference, overall effective amplification can be conducted. The detection kit, the primer group and a library building method can be applied to personalized medication gene detection, and guidance is provided for personalized medication.
Owner:CAPITALBIO GENOMICS
AFP (Alpha-Fetoprotein) testing kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
ActiveCN101819206AImprove accuracyHigh sensitivityBiological testingBlood specimenPrenatal screening
The invention relates to an alpha-fetoprotein testing kit (time-resolved fluoroimmunoassay) applicable for mid-pregnancy prenatal screening and a preparation method thereof. The kit comprises the following main components of an experimental buffer solution, a concentrated washing solution, an enhancement solution, a reaction plate, a standard product and a quality control product of filter paper dried blood spots and a europium marker. The method for preparing the kit according to the invention comprises the following steps of: 1. preparing the experimental buffer solution, the concentrated washing solution and the enhancement solution; 2. coating the reaction plate; 3. preparing the standard product and the quality control product; 4. preparing the europium marker; 5. separately loading; 6. sticking labels; and 7. assembling into a finished product. The invention has the characteristics that the accuracy and the sensitivity of a detected result are high, the stability is good, and a detecting method is economical, convenient and safe and has no traumatic property, has high automation degree, and the like. A filter paper dried blood spot technology is applied to prenatal screening work, which is beneficial to blood specimen collection, storage and transportation and ensures the experimental reliability.
Owner:GUANGZHOU FENGHUA BIOENG
Biological sample collection and preservation
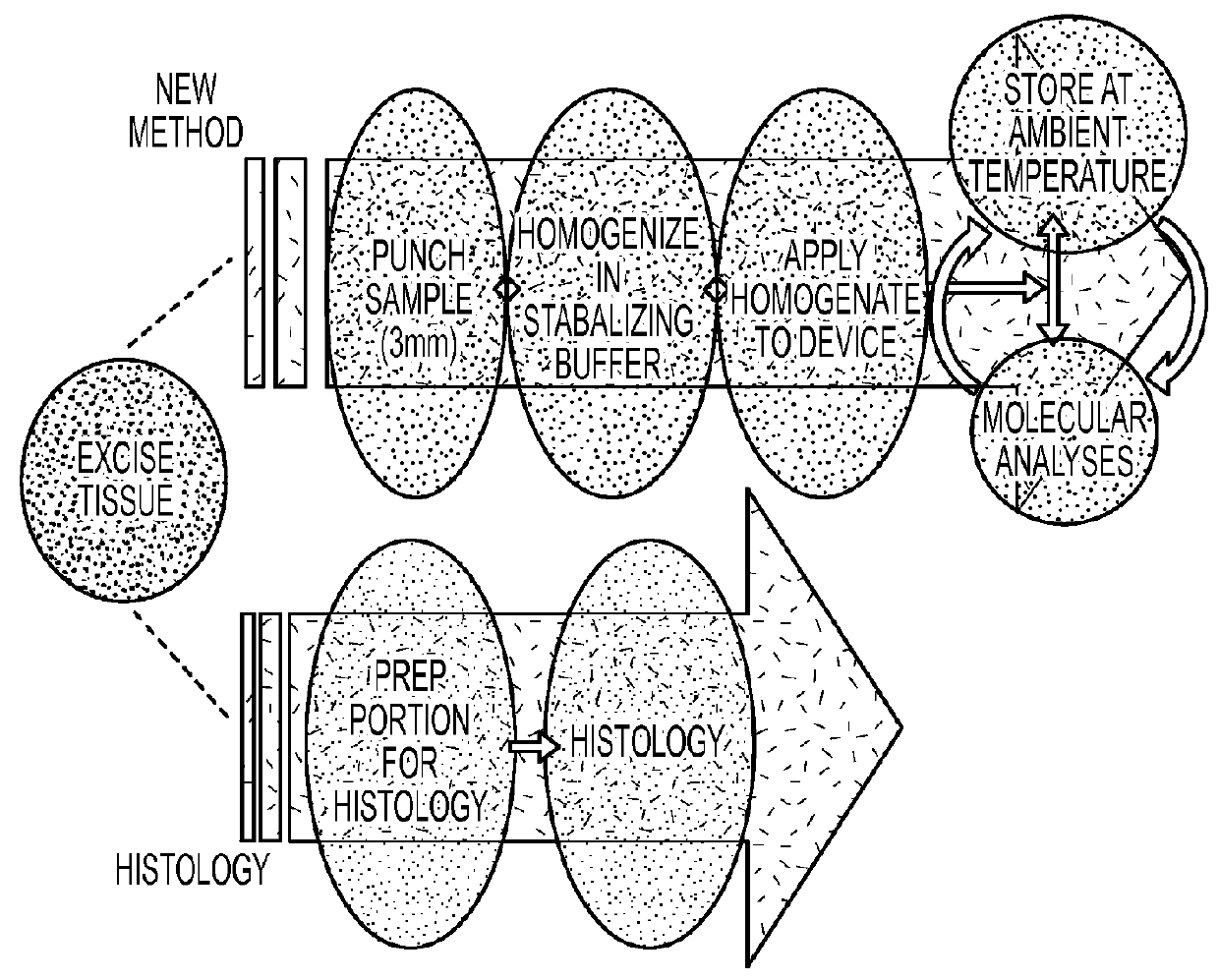
An embodiment of the claimed invention is directed to a method that greatly streamlines and reduces costs for tissue preparation, preservation, long-term storage and sample retrieval for molecular analysis using a method based on dried blood spot (DBS) technology. In this method, a small needle punch sample of freshly excised tissue will be homogenized in stabilizing reagent and inserted into a device containing absorbent material and drying agent. This device is suitable for long-term sample storage at ambient temperature and allows for easy removal of sections for biomarker analysis.
Owner:SPOT BIOSCI LLC
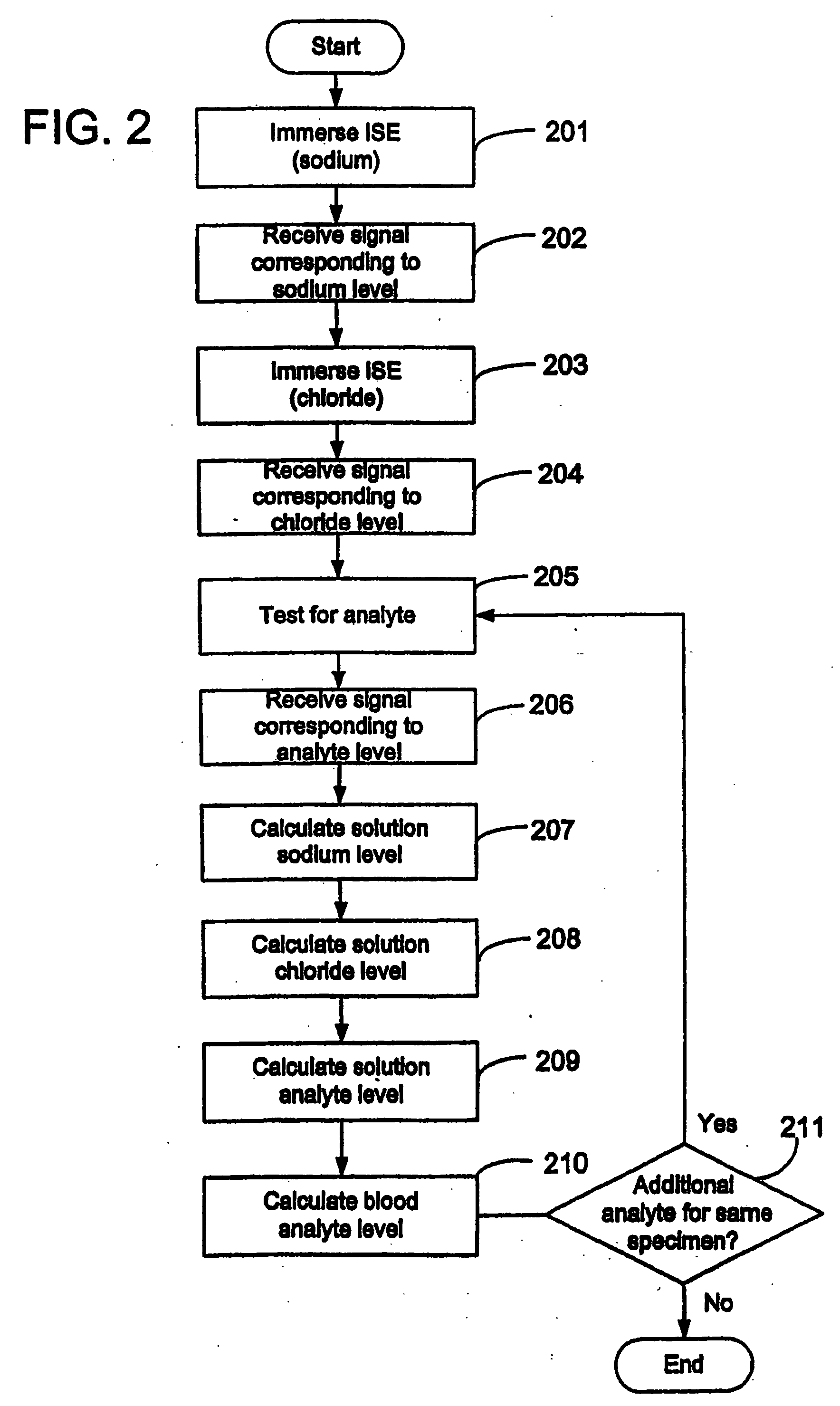
Quantitative analysis of a biological sample of unknown quantity
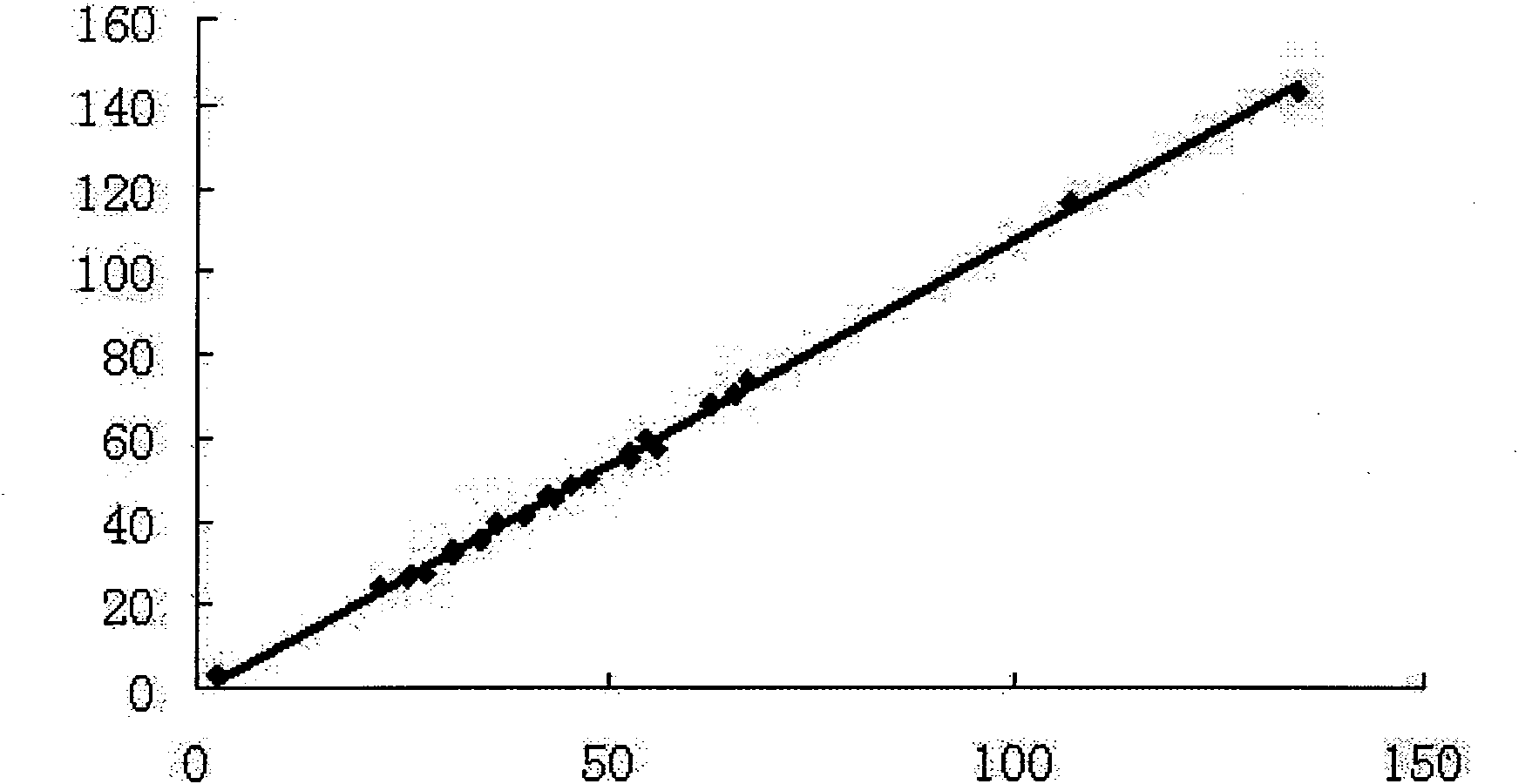
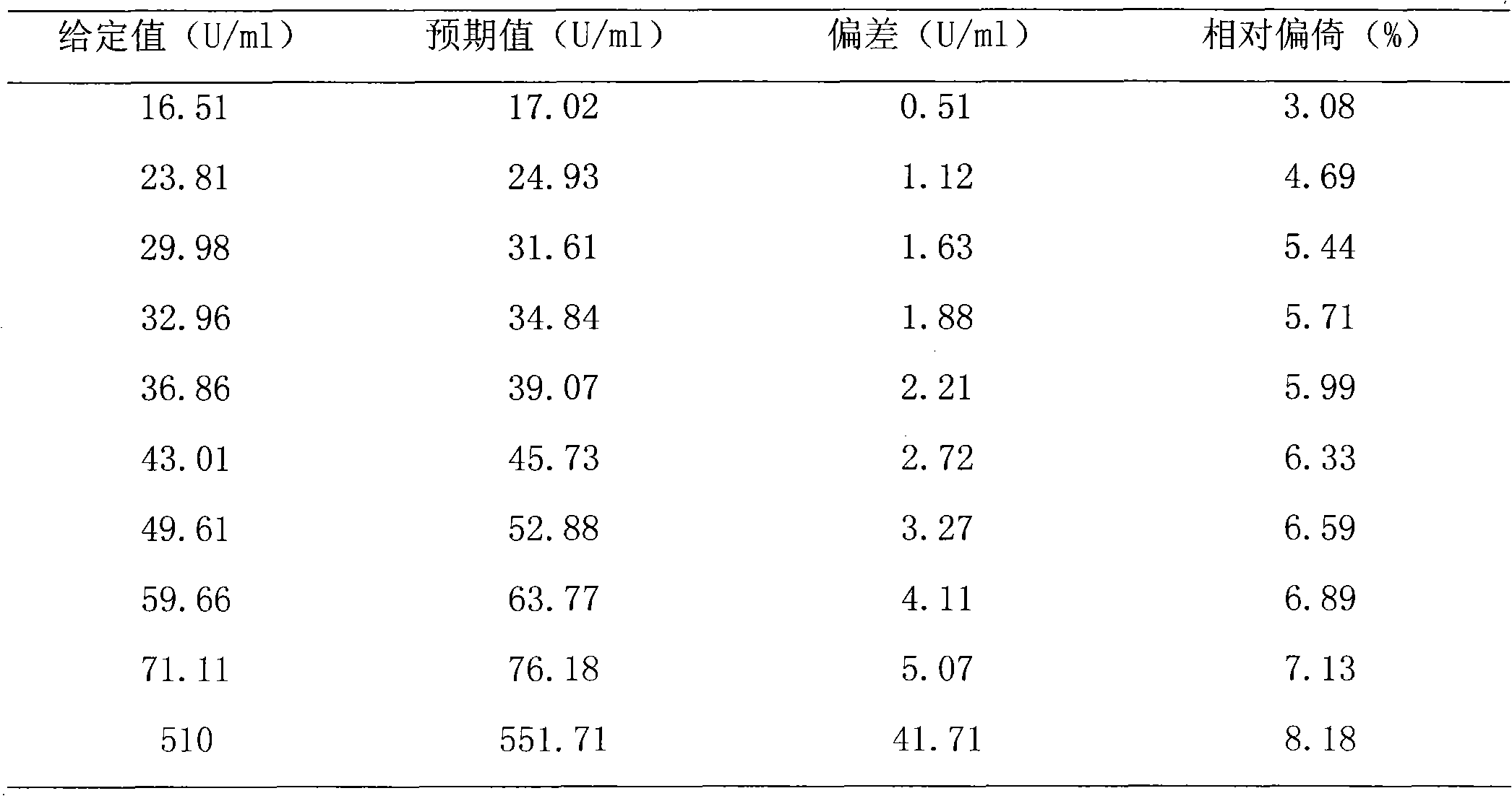
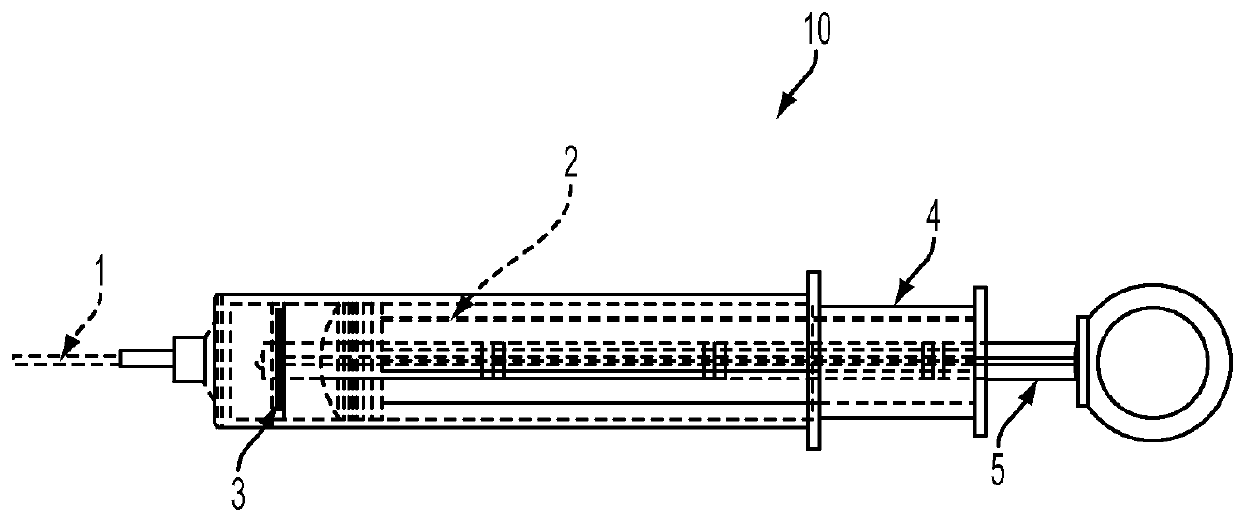
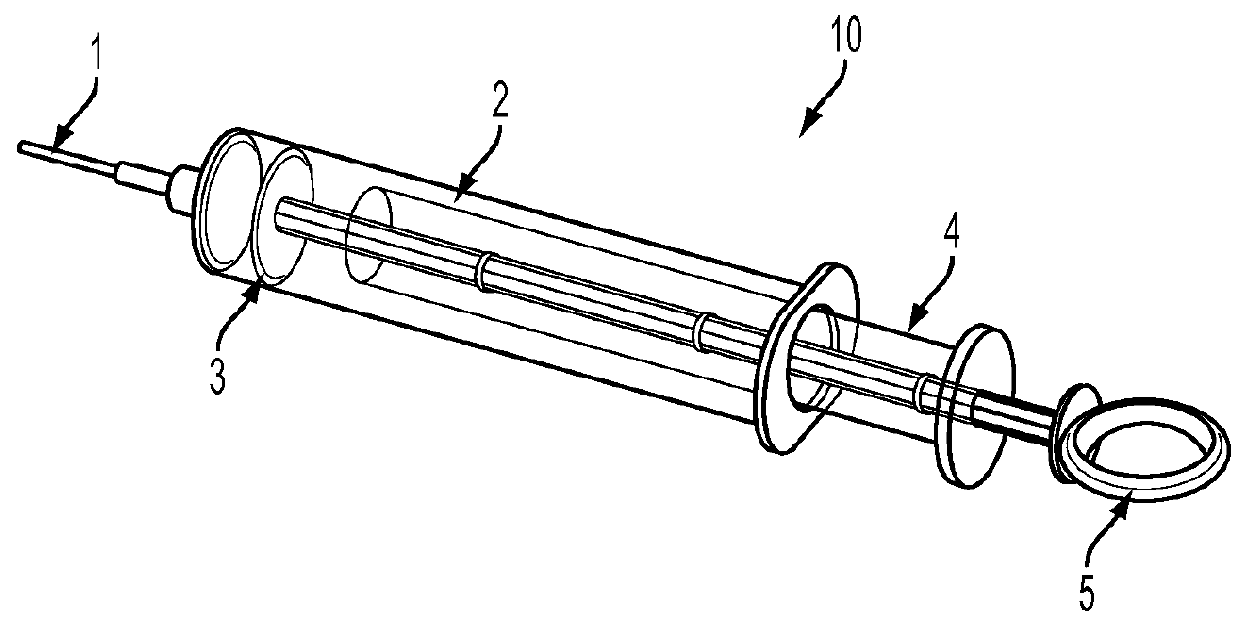
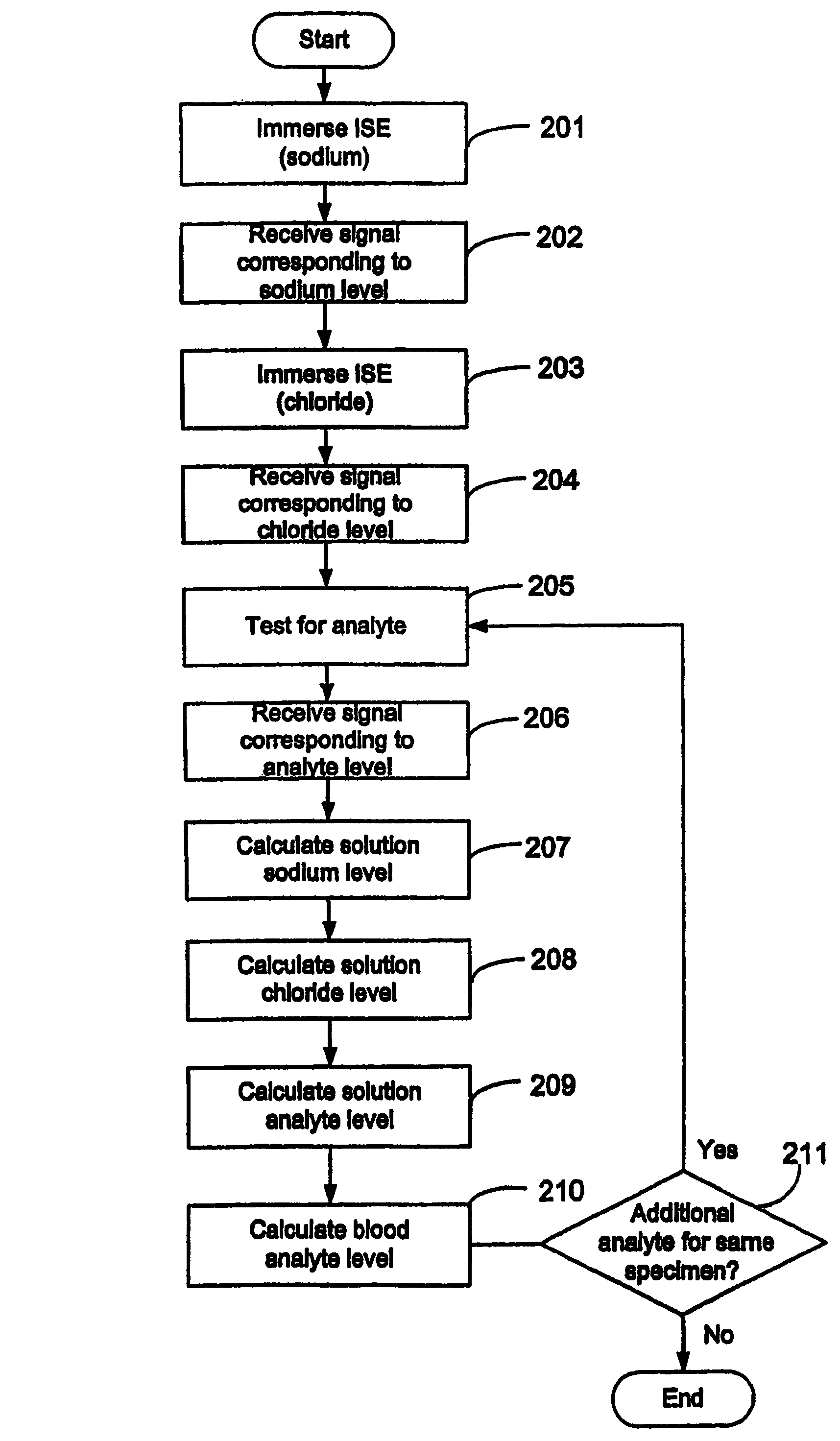
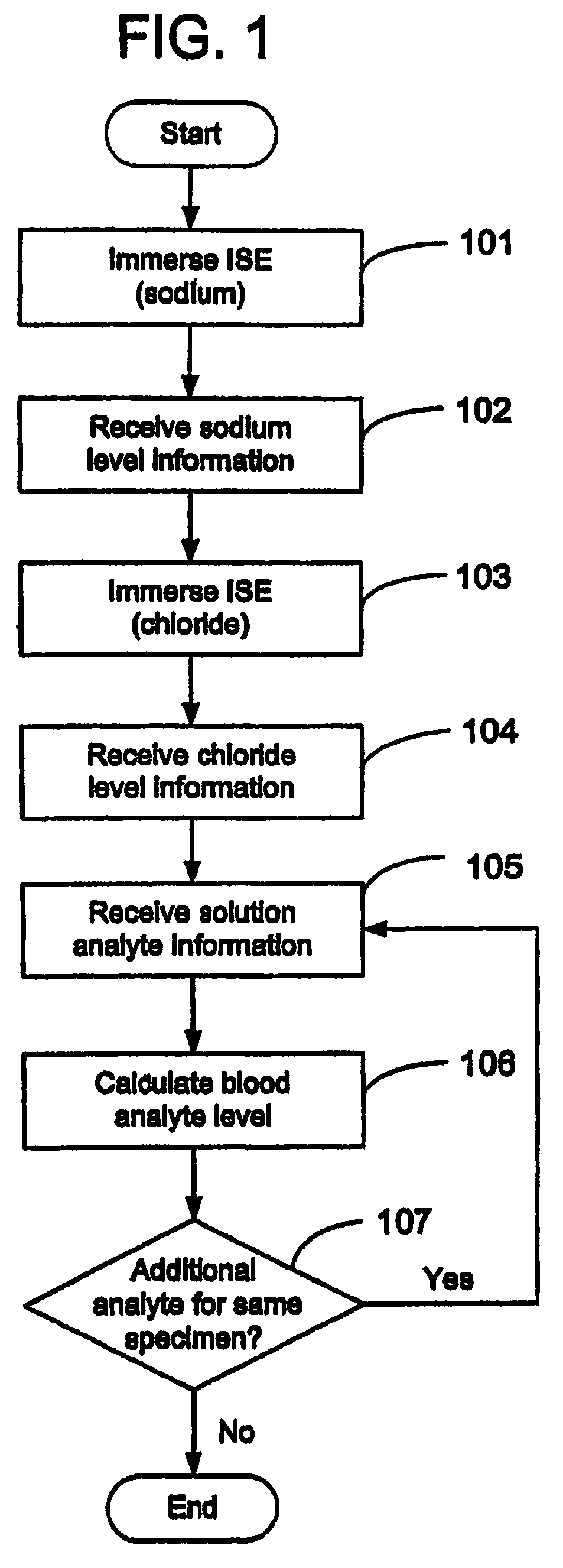
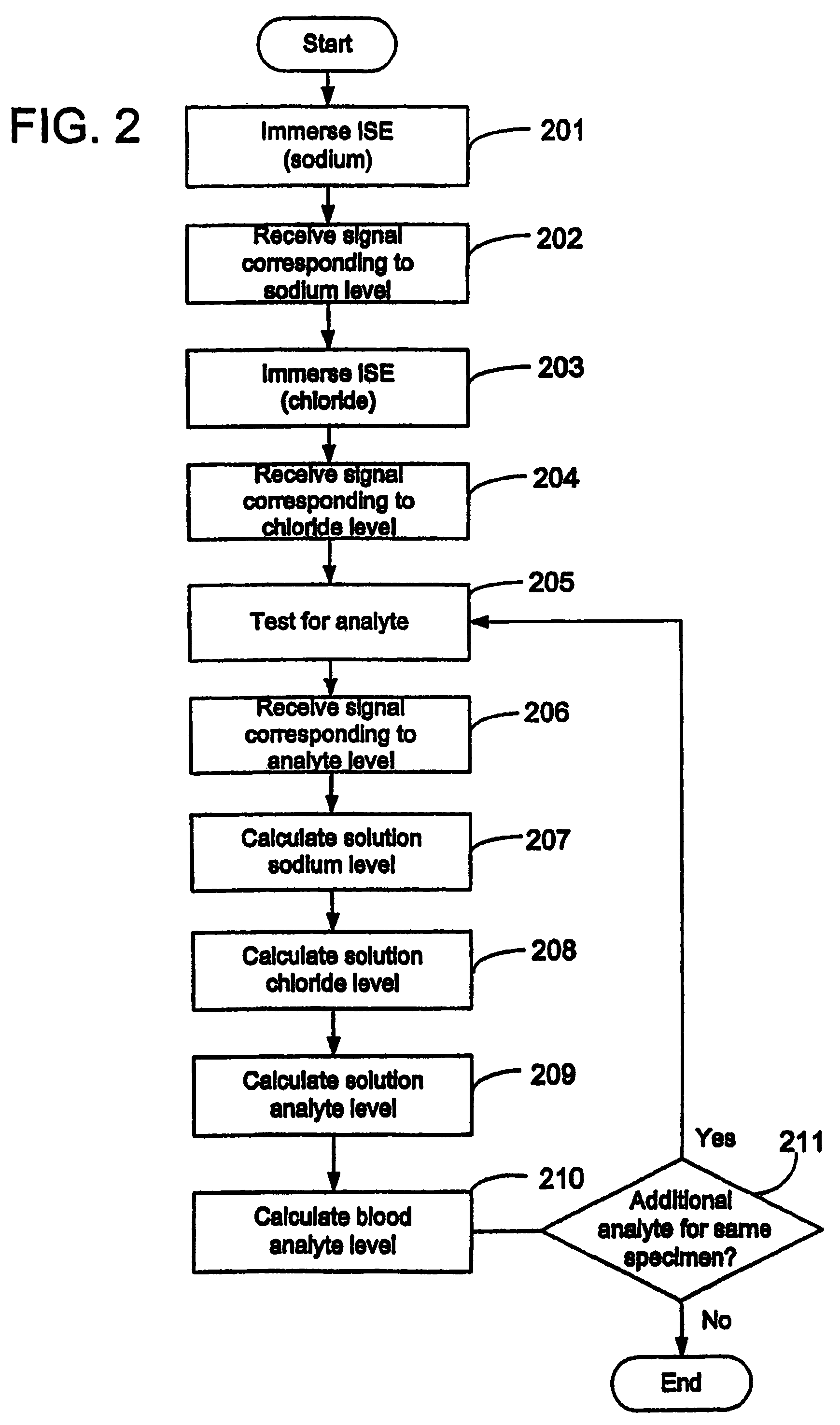
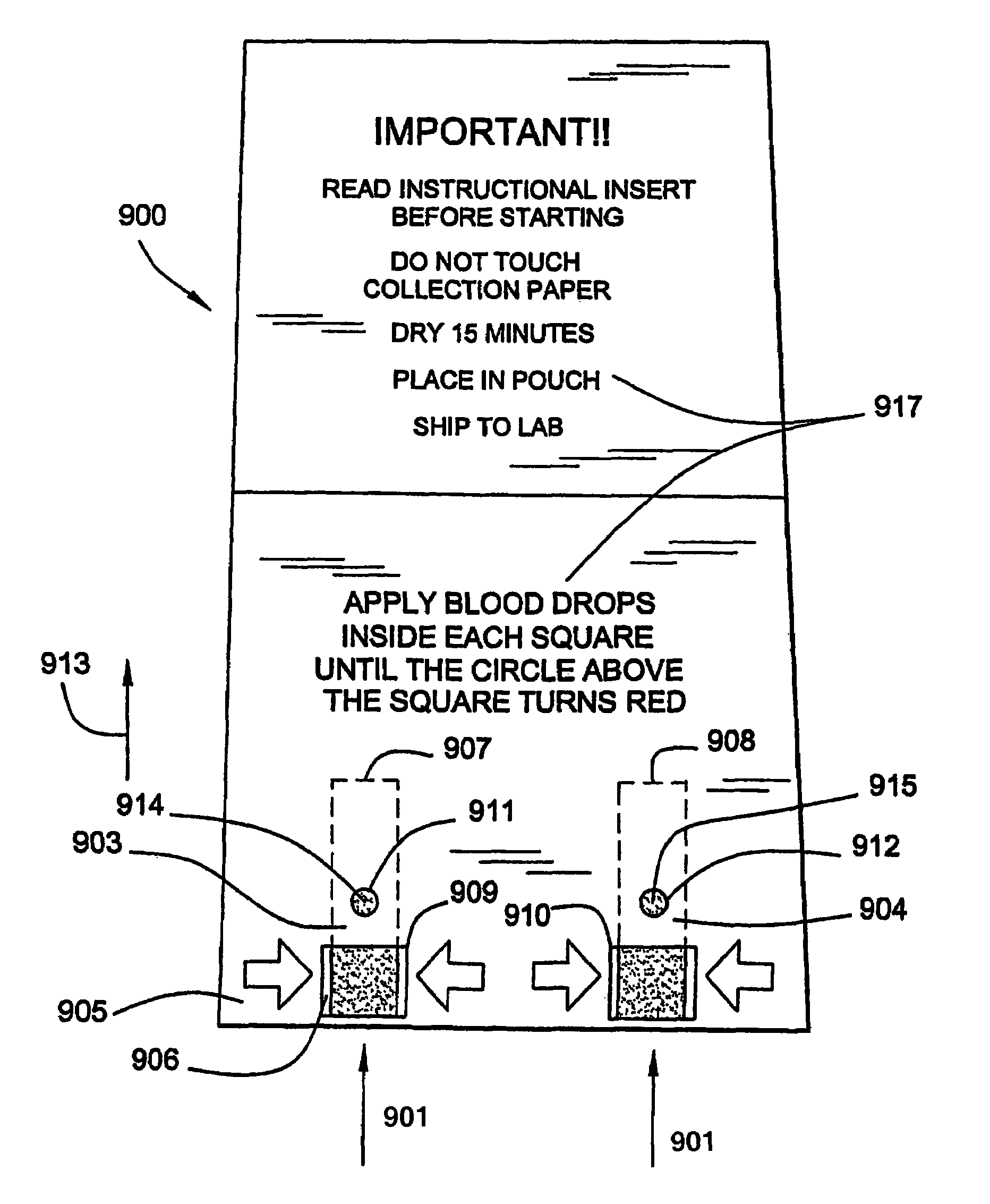
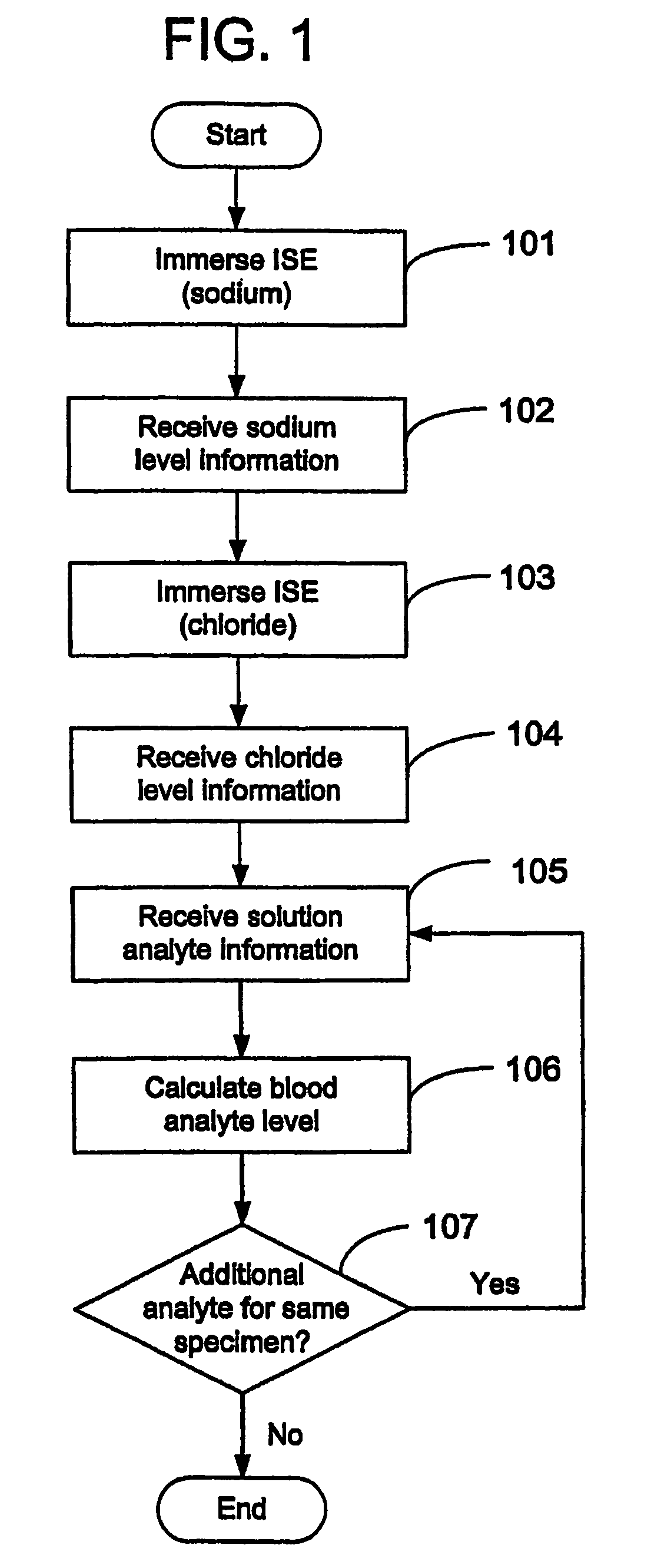
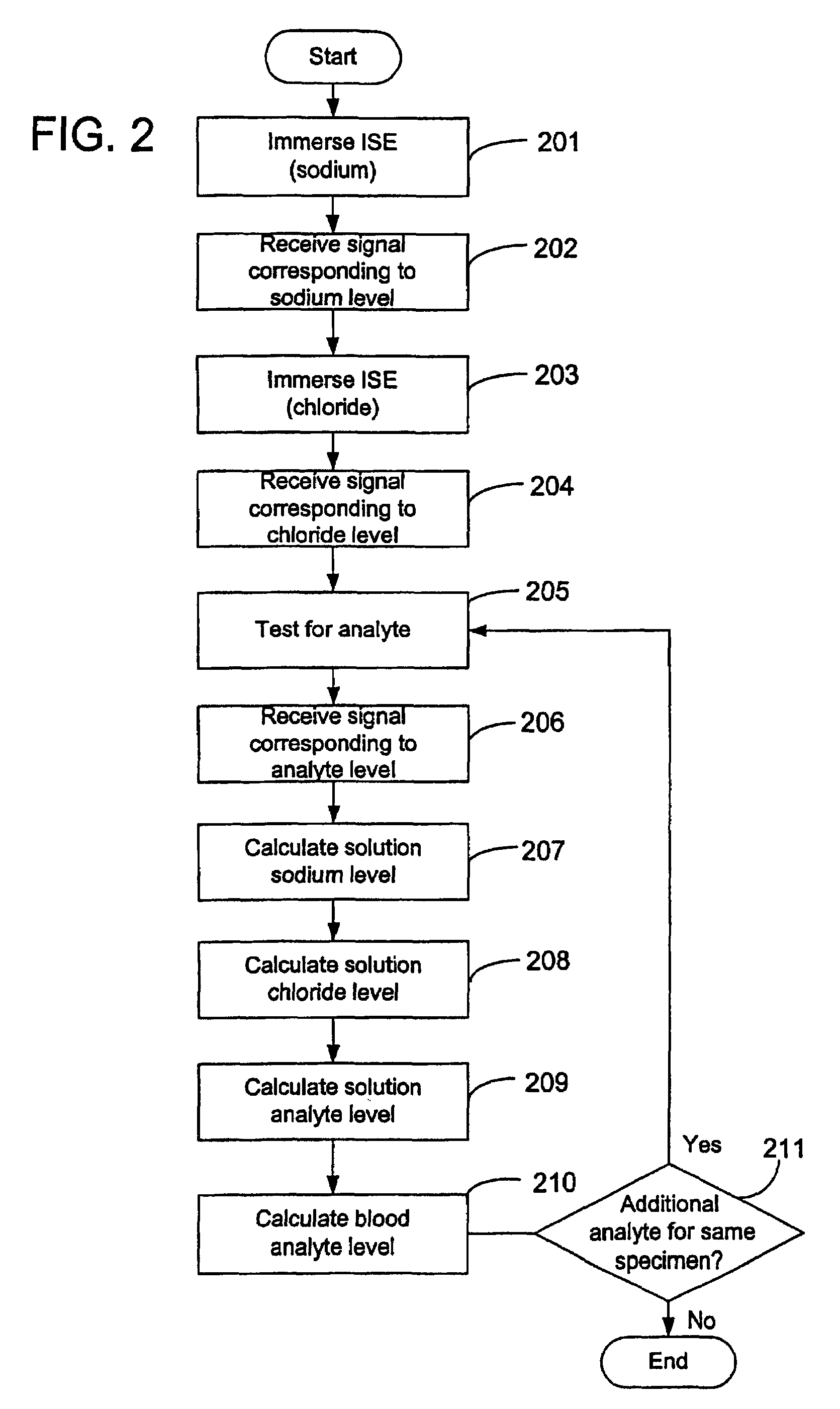
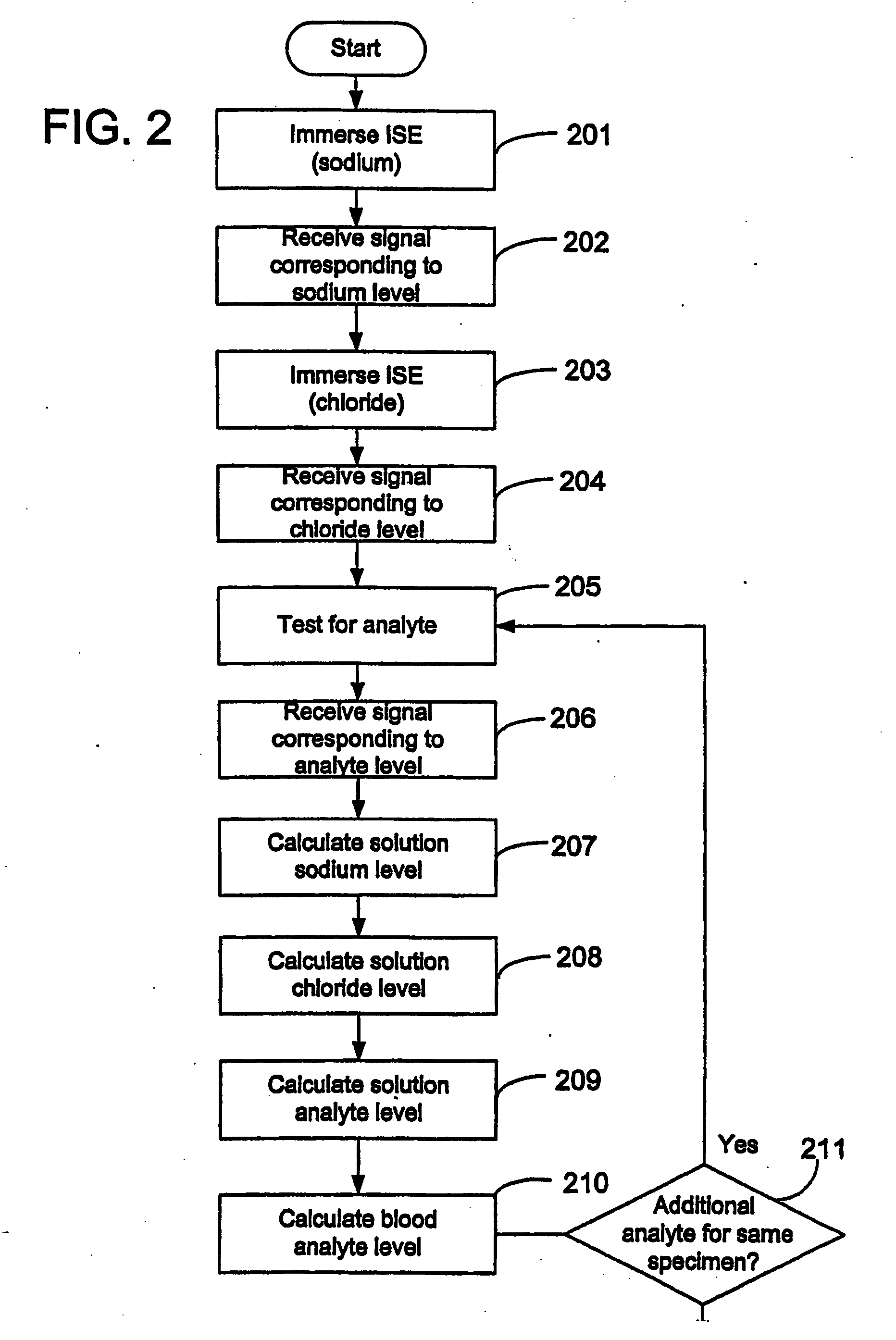
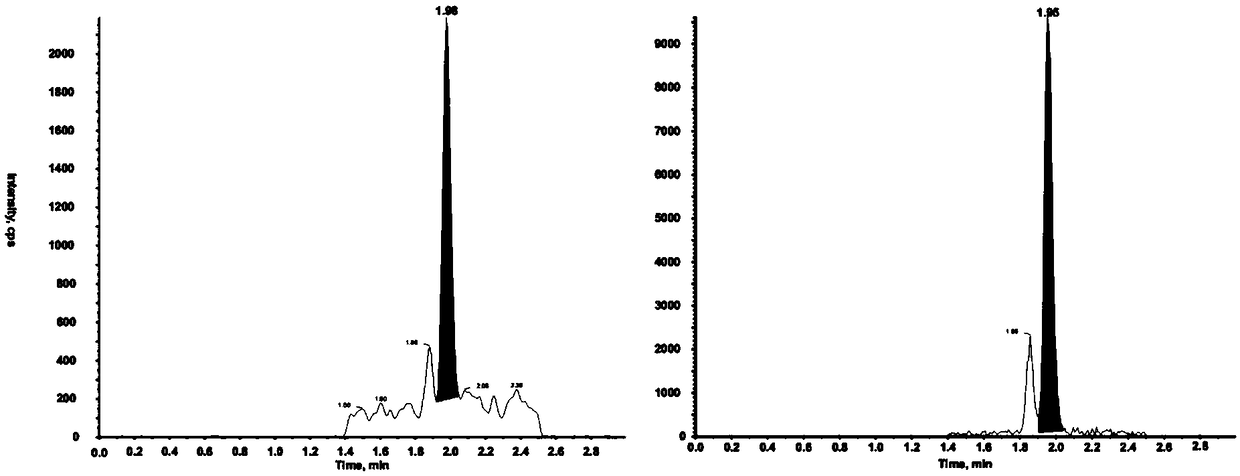
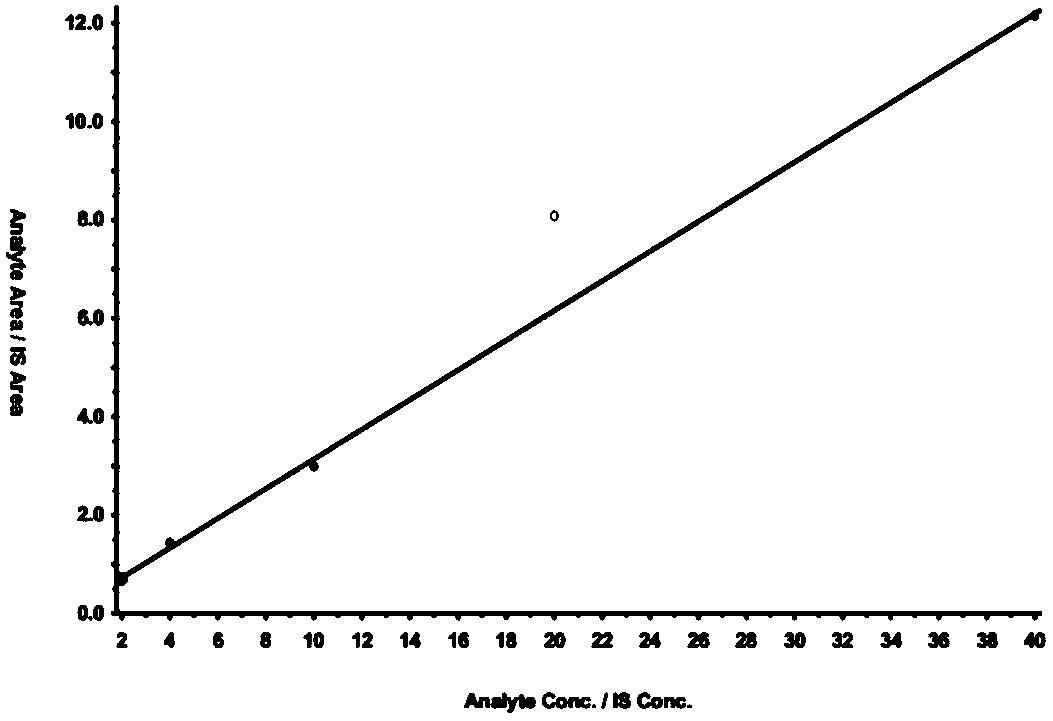
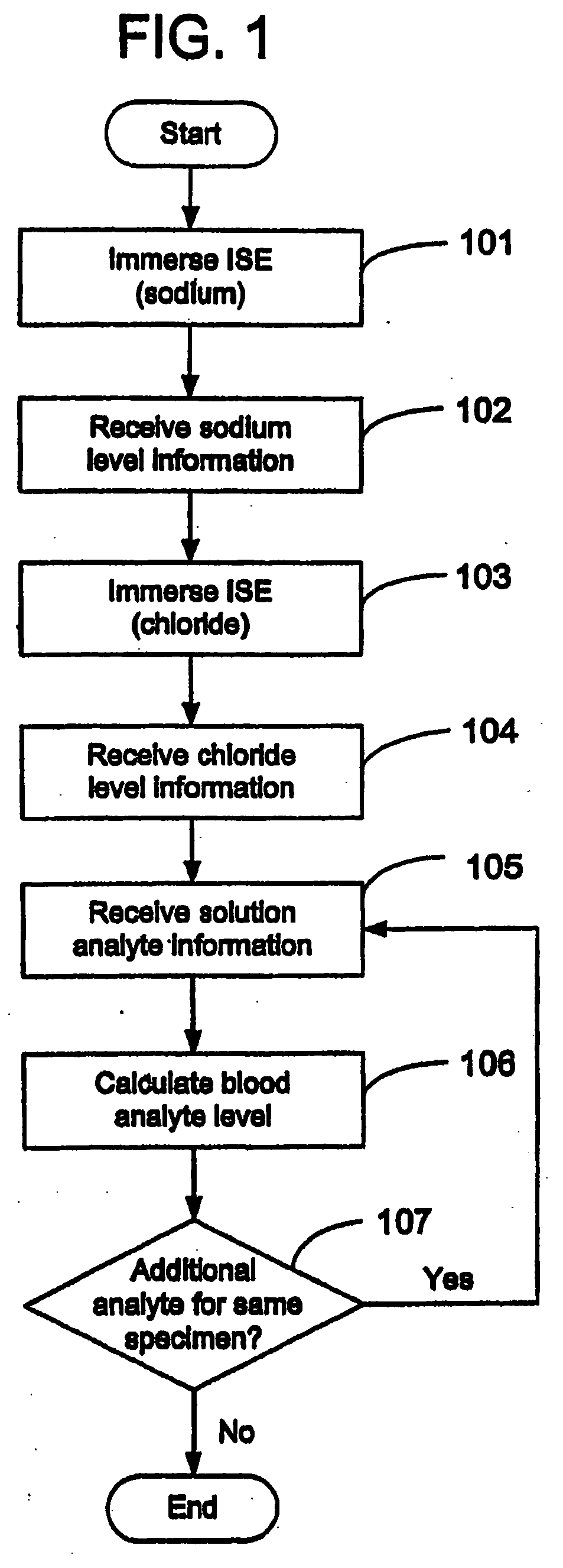
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Quantitative analysis of a biological sample of unknown quantity
ActiveUS7611670B2Analysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
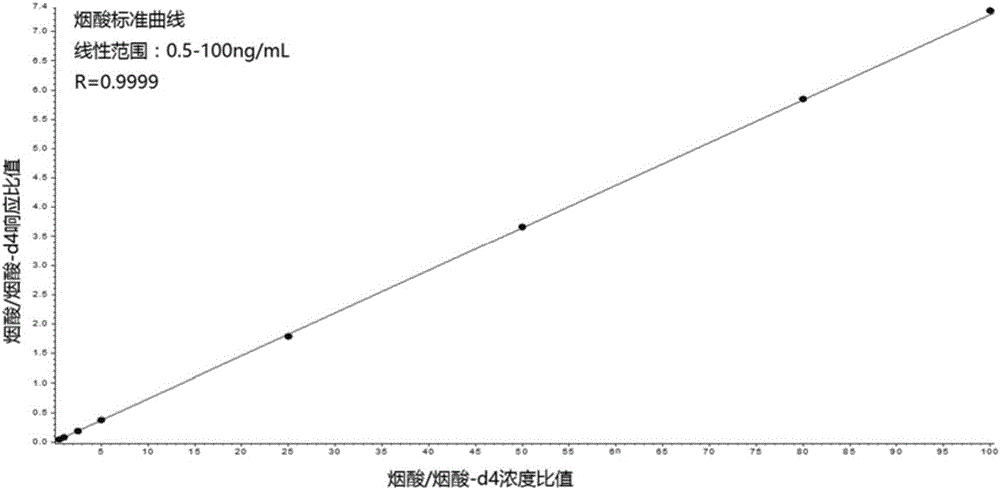
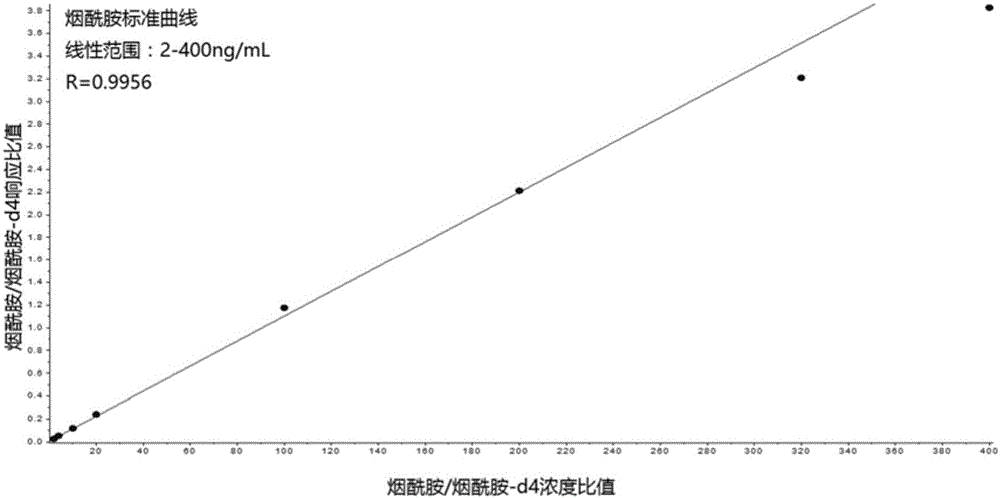
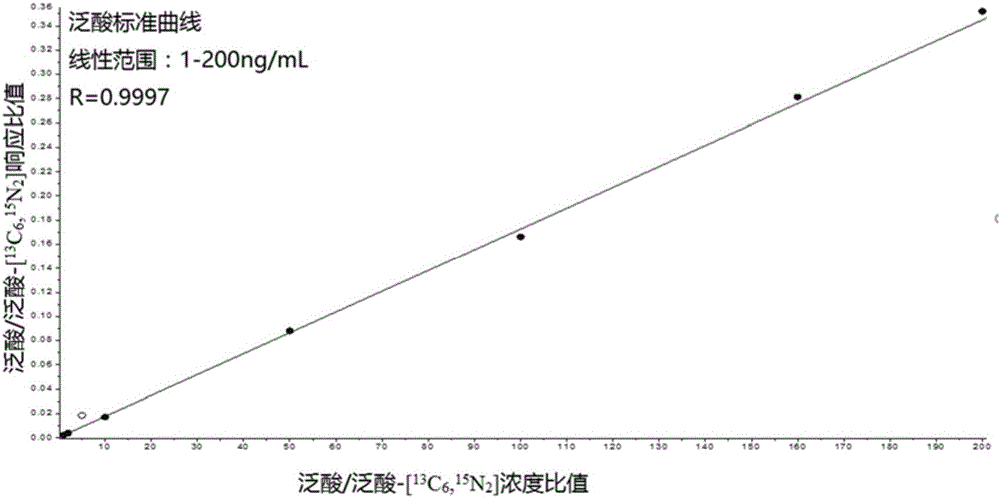
Kit for simultaneously quantifying and detecting niacin, nicotinamide and pantothenic acid
InactiveCN105954453AGood precisionImprove accuracyComponent separationBiological testingBlood Collection TubeSmall sample
The invention provides a kit for quantitatively detecting niacin, nicotinamide and pantothenic acid in peripheral whole blood at the same time, which mainly consists of dried blood slices (Whatman protein saver 903), capillary blood collection tubes, nicotinic acid and nicotinamide and pantothenic acid vitamin standard substance and its isotope internal standard, trichloroacetic acid, and also includes dry blood spot preparation, dry blood spot pretreatment and detection steps. The nicotinic acid, nicotinamide, and pantothenic acid dry blood spot quantitative kit provided by the present invention combines dry blood spot sampling technology with high performance liquid chromatography-tandem mass spectrometry technology for the quantification of B vitamins, a minimally invasive, minimal blood collection at the same time Realize the evaluation of the nutritional status of multiple B vitamins, and realize the quantitative work of simultaneous detection of multiple vitamins under low sample volume conditions that cannot be accomplished by metabolic enzyme-dependent methods and immunoassay methods.
Owner:首都儿科研究所 +1
Quantitative analysis of a biological sample of unknown quantity
ActiveUS20050130310A1Simple methodAnalysis using chemical indicatorsMicrobiological testing/measurementAnalyteCholesterol
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Newborn total galactose detection kit, as well as application method and preparation method thereof
The invention discloses a newborn total galactose detection kit. The newborn total galactose detection kit comprises mixed freeze-dried powder, freeze-dried galactose dehydrogenase powder, galactose filter paper dried-blood spot calibration product, a quality control product, copper reagent and a white reaction board, wherein the mixed freeze-dried powder comprises an experiment buffer solution, alkaline phosphatase and oxidized form coenzyme II. The invention further discloses an application method and a preparation method of the newborn total galactose detection kit. The newborn total galactose detection kit is economical, has the time-saving and labor-saving effects, can be used for screening galactosemia of newborns, and lays a good technological base for the promotion of the screening for the newborns.
Owner:GUANGZHOU FENGHUA BIOENG
Primers, probes, kit and method for detecting polymorphism of human CYP2C19 gene
InactiveCN106755535AReduce signal strengthStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr method
The invention belongs to the technical field of gene engineering and discloses a composition of primers and probes for detecting polymorphism of human CYP2C19 gene, a kit containing the composition of the primers and the probes, and a fluorescence PCR method for detecting the polymorphism of the human CYP2C19 gene by using the composition of the primers and the probes or the kit. Based on a TaqMan fluorescence PCR technology, the primers, the probes and the kit are simple and rapid, and are high in sensitivity; in addition, through reasonable collocation of the primers and the probes, the interaction between the primers, the interaction between the primers and the probes and the interaction between the probes can be effectively avoided; the detection errors are reduced. When the primers, the probes and the kit, provided by the invention, are used for detecting the polymorphism of the human CYP2C19 gene, the primers, the probes and the kit has the advantages that the sensitivity is high, the specificity is high, the operation is simple, rapid and safe, the result is simply and intuitively determined and read, and blood samples or dried blood spots samples on filter paper can be directly used as templates.
Owner:SHANGHAI TISSUEBANK MEDICAL LAB CO LTD +3
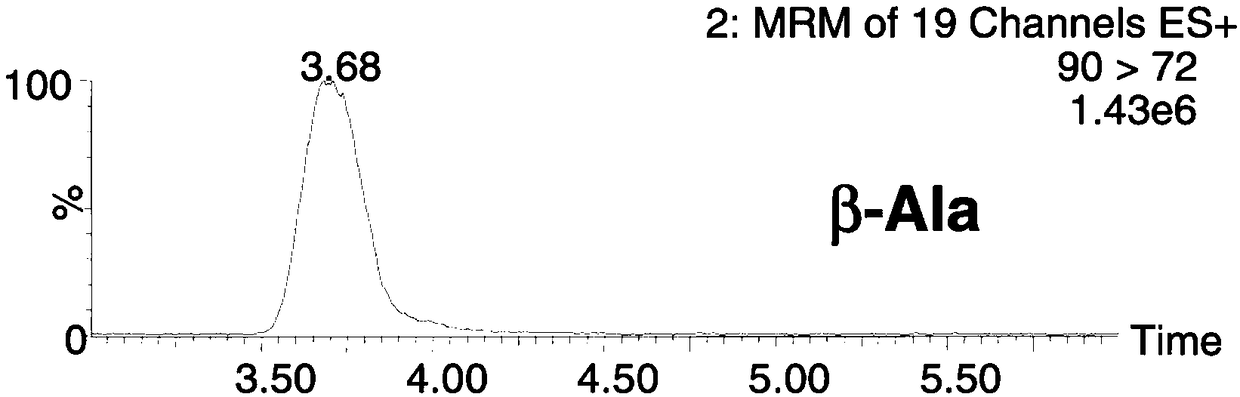
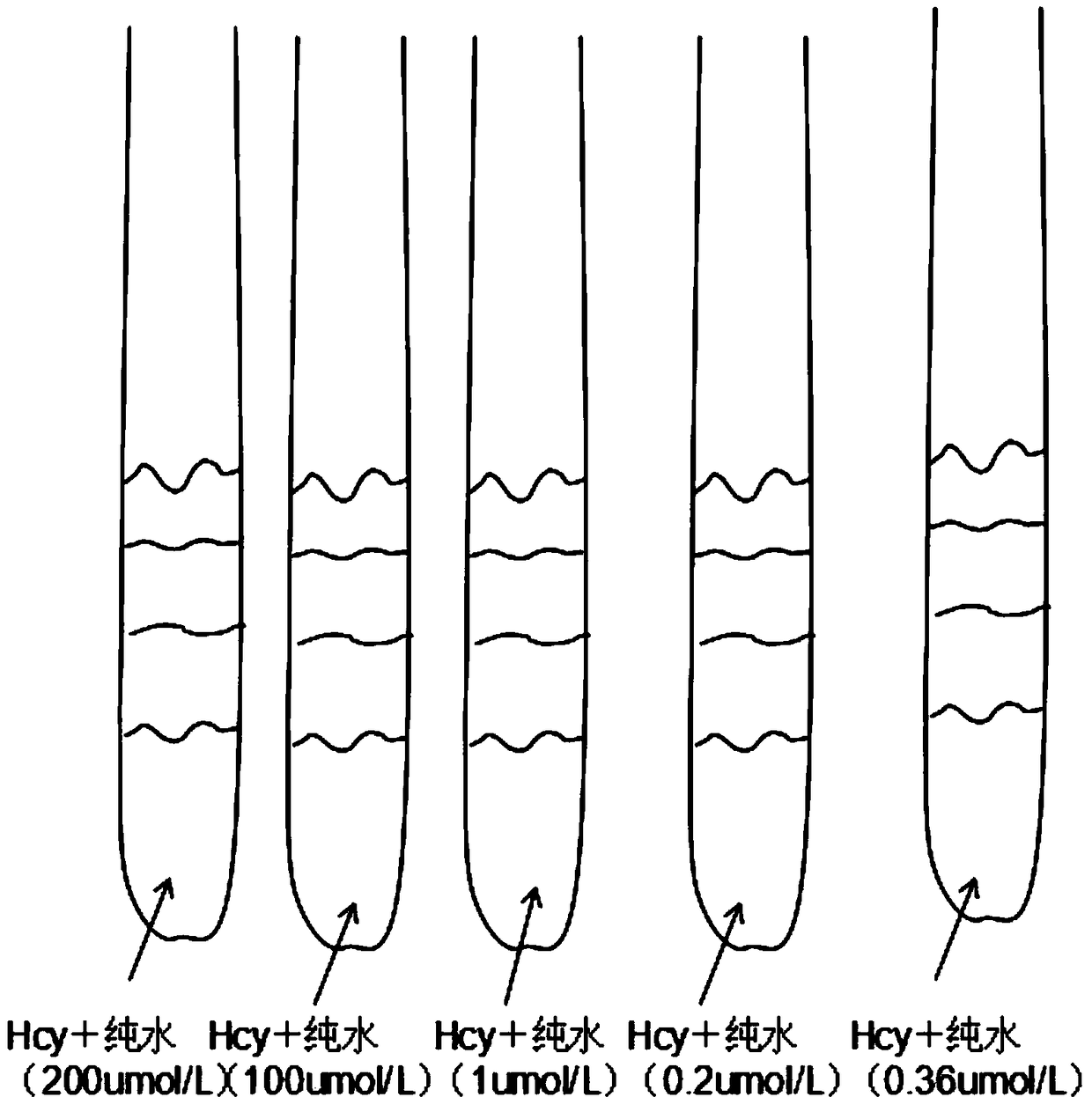
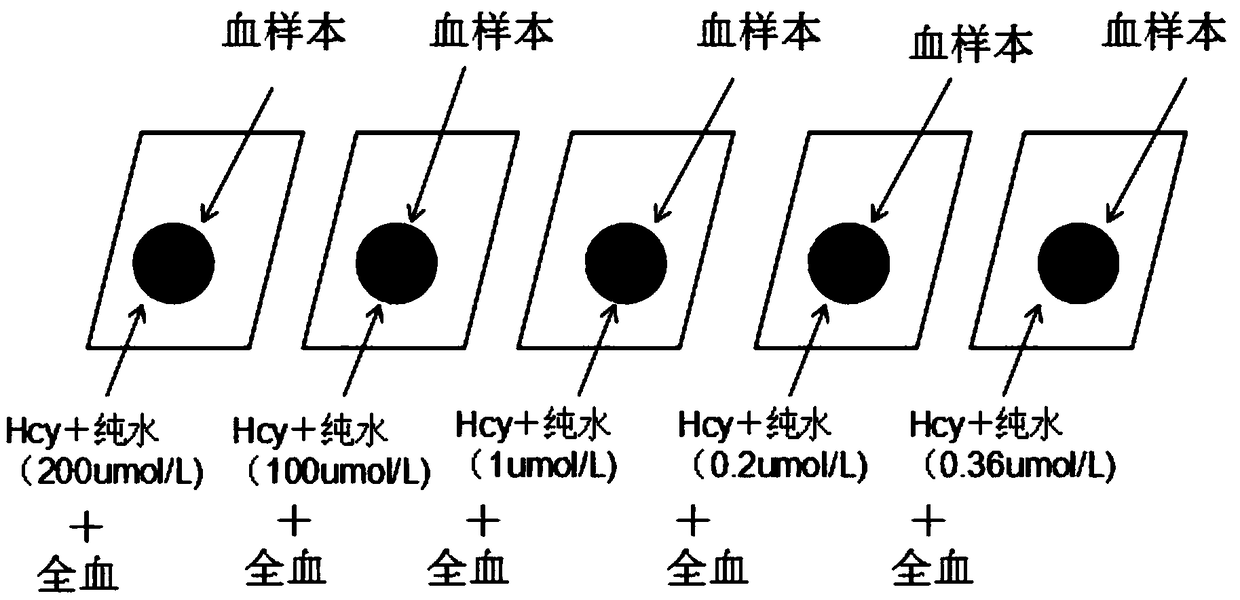
Rapid measurement method for content of homocysteine in dried blood spot
The invention discloses a rapid measurement method for content of homocysteine in a dried blood spot and applies to content measurement of homocysteine in a dried blood spot sample. The method comprises the following steps: obtaining a quantitative standard correction curve of homocysteine; preparing a sample for measurement; performing high-throughput liquid chromatographic separation on homocysteine; detecting homocysteine with tandem mass spectrometry; obtaining content of homocysteine. The method has the advantages of short measurement time, high throughput, high detection sensitivity, high specificity and relatively simple pretreatment process, and has a significance in clinical disease diagnosis and screening.
Owner:HANGZHOU BAICHEN MEDICAL LAB CO LTD +1
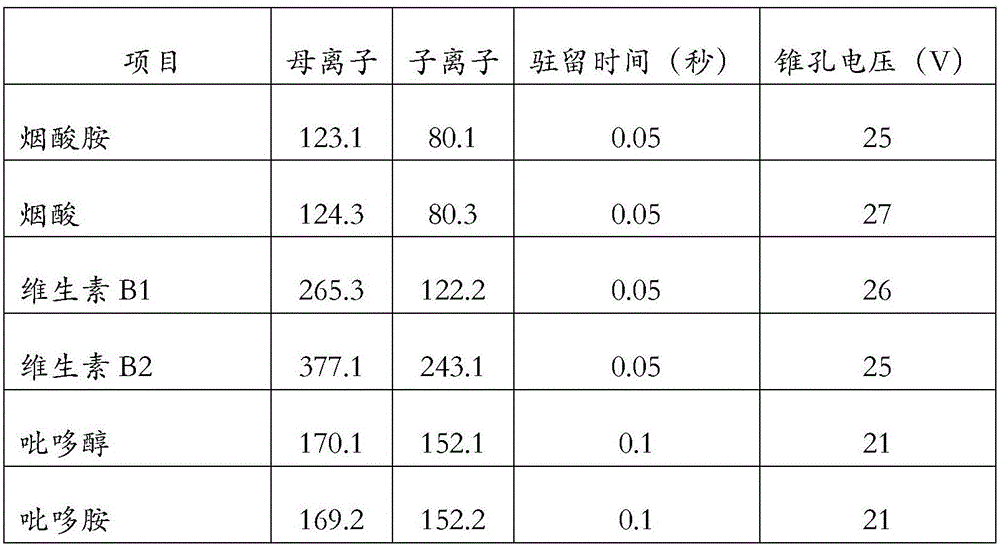
Method and kit for simultaneously detecting multi vitamins in dry blood spot card
The invention discloses a method for simultaneously detecting multi vitamins in a dry blood spot card. The method comprises the following steps: (1) mixing a dry blood spot card specimen and an extracting solution; oscillating and incubating; then centrifuging and taking supernatant; (2) after uniformly mixing the supernatant obtained by the step (1) with a mobile phase and an internal standard mixed solution, detecting by adopting a tandem mass spectrometry; comparing ion intensity of the vitamins and internal standard of the vitamins to obtain the content of the vitamins in blood serum to be detected or blood plasma to be detected. Furthermore, the invention also discloses a kit for simultaneously detecting the multi vitamins in the dry blood spot card; the kit comprises the internal standard mixed solution, the extracting solution, the mobile phase and a probe washing solution. According to the method disclosed by the invention, multi water-soluble and fat-soluble vitamins are extracted from the dry blood spot card and more than ten vitamins in one specimen can be simultaneously quantified within 2min to 3min by utilizing a tandem mass spectrometer; a detection period is remarkably shortened, and a detection result of the vitamins has higher precision and accuracy.
Owner:GUANGZHOU FENGHUA BIOENG
Detection method of hormone in blood
The invention provides a detection method of hormones in blood, and belongs to the field of the detection of the hormones. The detection method comprises the following steps of mixing and storing the blood and an internal standard solution including a hormone isotope internal standard on a filter paper disc, and preparing an obtained mixture into a dried blood spot. An extracting solution is obtained from the dried blood spot through extraction, and a detection solution is obtained by carrying out derivatization treatment on the extracting solution. The hormone in the detection solution is detected through liquid chromatographic-mass spectrometric detection. In the method provided by the invention, the blood is stored on the filter paper disc, and then the purpose of quickly and effectively carrying out qualitative and quantitative detection on the hormone in the blood is realized through extraction and derivatization processes and by utilizing a liquid chromatographic-mass spectrometric method. As the blood is stored on the filter paper disc and is stored in the form of a solid, a biological safety problem possibly brought about in a transportation process is avoided; moreover, the stability can be greatly improved; therefore, a detected sample is prevented from being polluted by a microorganism.
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
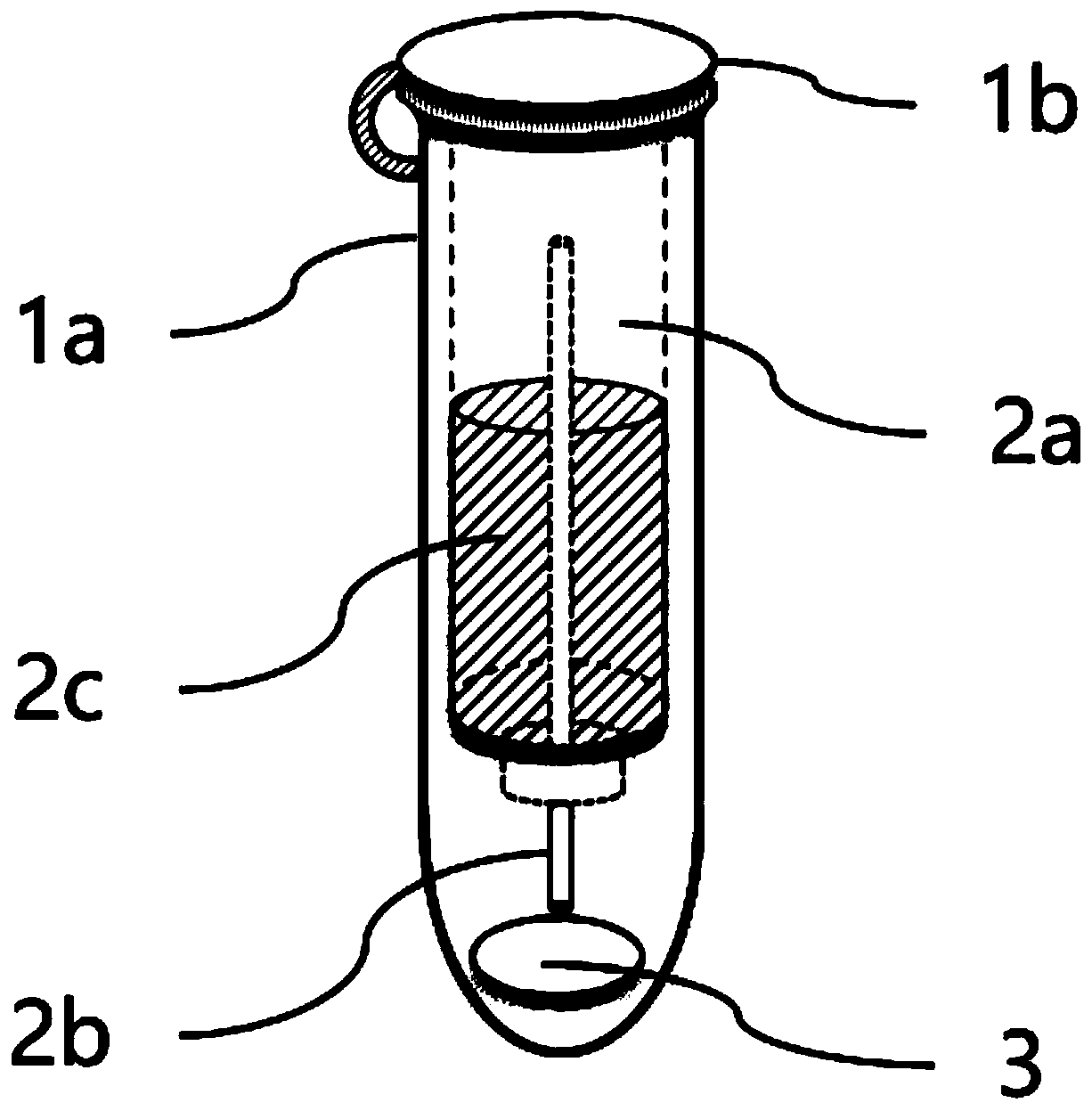
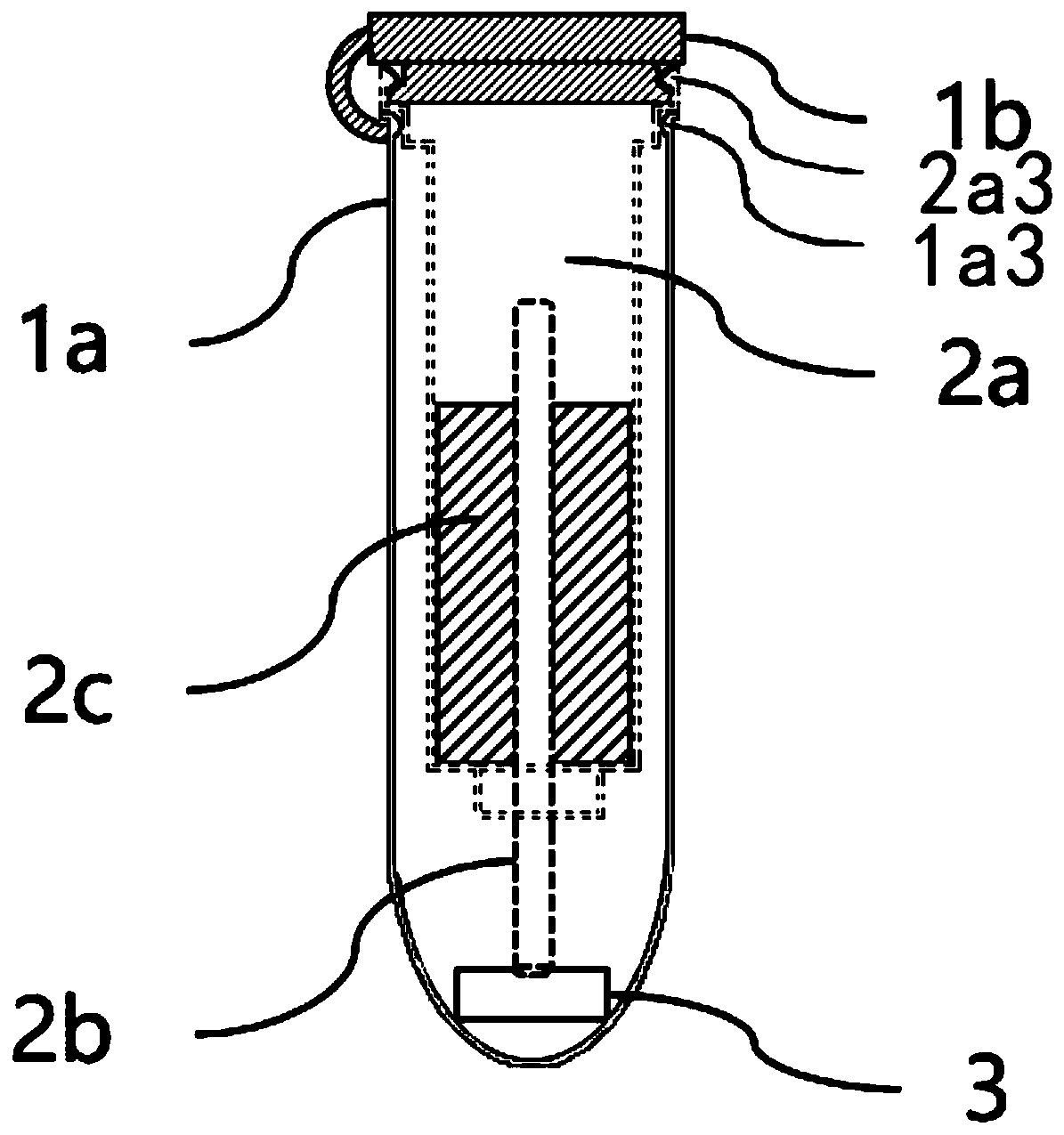
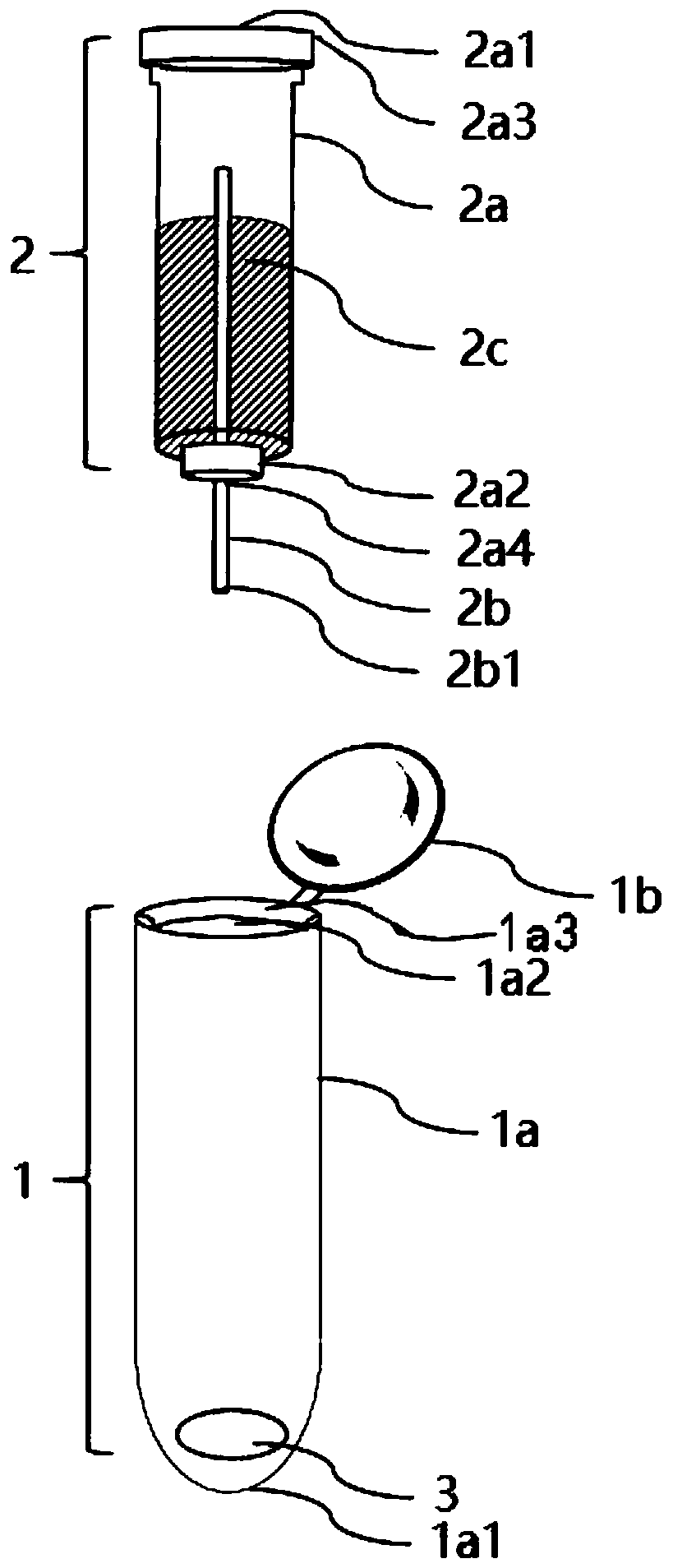
Dried blood spot quantitative collection device and method
PendingCN111474257AQuantitative results are accurateLow blood volumeComponent separationMicrobiological testing/measurementBlood Collection TubeCapillary Tubing
The invention provides a dried blood spot quantitative collection device. The dried blood spot quantitative collection device comprises a sample collection tube, sample collection filter paper and a quantitative blood collection tube, wherein the sample collection tube comprises a tube body and a tube cover; the tube body is a straight tube, and the tube cover is used for sealing the tube opening.The quantitative blood collection tube comprises a quantitative capillary tube and a fixing piece; the quantitative capillary tube is fixed on the fixing piece and is provided with a blood sampling end which is used for sampling blood and is exposed out of the fixing piece; the fixing piece is clamped in the tube opening, and the quantitative capillary tube extends into the tube body; the samplecollection filter paper is arranged in the tube body, and the blood sampling end abuts against the sample collection filter paper. The invention further provides a method for collecting the dried blood spots. The integrated blood collecting device design is adopted, sample collection, drying, transportation, storage and extraction can be achieved in the same device, sample pollution is avoided, and the accuracy of a dried blood spot quantitative detection result is improved.
Owner:WUXI DIAGNOSTICS LAB SHANGHAI CO LTD +1
High-performance liquid chromatography-tandem mass spectrometry detection method of 25-hydroxy vitamin D in dried blood spots
The invention discloses a high-performance liquid chromatography (HPLC)-tandem mass spectrometry detection method of 25-hydroxy vitamin D in dried blood spots. The method comprises the following steps: (1) ultrasonic extraction: taking a dried blood spots sample, adding an extraction solvent, ultrasonically extracting, and then adding an internal standard working solution, performing vortex-mixing, centrifuging, taking upper extraction liquid, drying through nitrogen; (2) derivatization reaction: adding the sample processed in the step (1) into a PTAD derivatization working solution to redissolve, reacting at constant temperature, and adding the ethanol to terminate the reaction, and drying through the nitrogen; and (3) redissolving: performing analysis detection by adopting the HPLC-tandem mass spectrometry. The method disclosed by the invention is high in efficiency, high in flux, low in cost, simple for operation, high in accuracy, and high in sensitivity, free form conflict limitation on other detection projects, and suitable for fast detecting batch of clinical samples; and meanwhile, the wound on the subject is small by adopting the dried blood spots method, the amount of collecting blood is less, the sample is convenient for collection and transportation, and easy to save, the stabiligy is good, and the method is especially suitable for the detection on the population (such as infants) difficult for venous blood collection.
Owner:杭州凯莱谱精准医疗检测技术有限公司
Quantitative analysis of a biological sample of unknown quantity
Disclosed is a method for testing a modified specimen such as a dried blood spot, plasma or serum specimen, for an analyte of interest, such as cholesterol. In accordance with the disclosed subject matter, the level of the analyte of interest in the medium from which the modified specimen was obtained (e.g., from a patient's blood) is determined based on the level of an analyte in a solution formed from the modified specimen and on the level of at least one normalizing analyte. The analyte and normalizing analyte each may be an ion, compound, biochemical entity, or property of the specimen. Also disclosed are a fluid collector and a fluid collection device.
Owner:PWNHEALTH CHICAGO LLC
Method and kit for determining metabolites on dried blood spot samples
ActiveUS20120273671A1Easy to implementAvoid serious complicationsIsotope separationMass spectrometersMetaboliteDay of life
A method for individuating with high sensitivity and specificity ADA metabolites from dried blood spot. The method described herein can be used to extract Adenosine and Deoxyadenosine from a sample under conditions that permit concurrently extracting other metabolites, such as amino acids, free carnitine, or acylcarnitines. For example, harsh extraction conditions (such as extreme acidity and high temperature) can be avoided. The method can be used, along with other neonatal screenings, on blood samples and preferably on dried blood spots (Guthrie cards) and more preferably on Guthrie cards obtained in the II-IV day of life. The method is reliable and reproducible, easy to perform and gives a definitive response within a short time (1-2 days). One or more kit for use in the method.
Owner:AZIENDA OSPEDALIERO UNIVRIA MEYER
Kit for screening and checking glucose-6-phosphate dehydrogenase (G6PD) deficiency of neonates and preparation method for kit
ActiveCN102519925AReduce false positive rateImprove the detection rateBiological testingFluorescence/phosphorescenceFull Term NeonateBiochemistry
The invention discloses a kit for screening and checking glucose-6-phosphate dehydrogenase (G6PD) deficiency of neonates and a preparation method for the kit. The kit for screening and checking the glucose-6-phosphate dehydrogenase (G6PD) deficiency of the neonates consists of substrate reagent dry powder, a substrate redissolved reagent, a copper reagent, a reaction board and filter paper dried blood spot quality control material. The detection result of the kit is high in accuracy and sensitivity, and the kit has the advantages of high stability, economy, simplicity, high efficiency and thelike; when the kit is applied to screening and checking for the neonates, the relevance ratio of heterozygote is improved; and the judgment accuracy is improved.
Owner:GUANGZHOU FENGHUA BIOENG
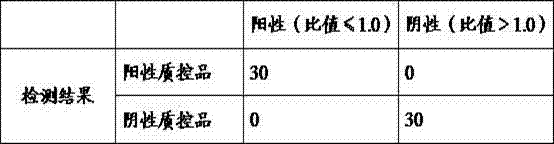
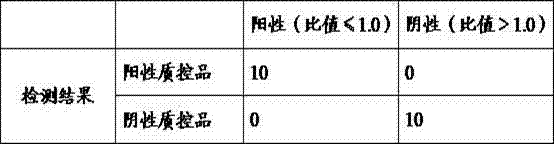
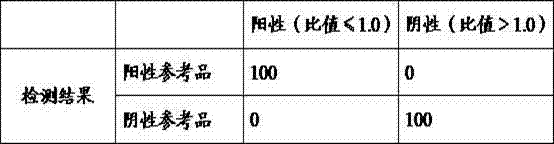
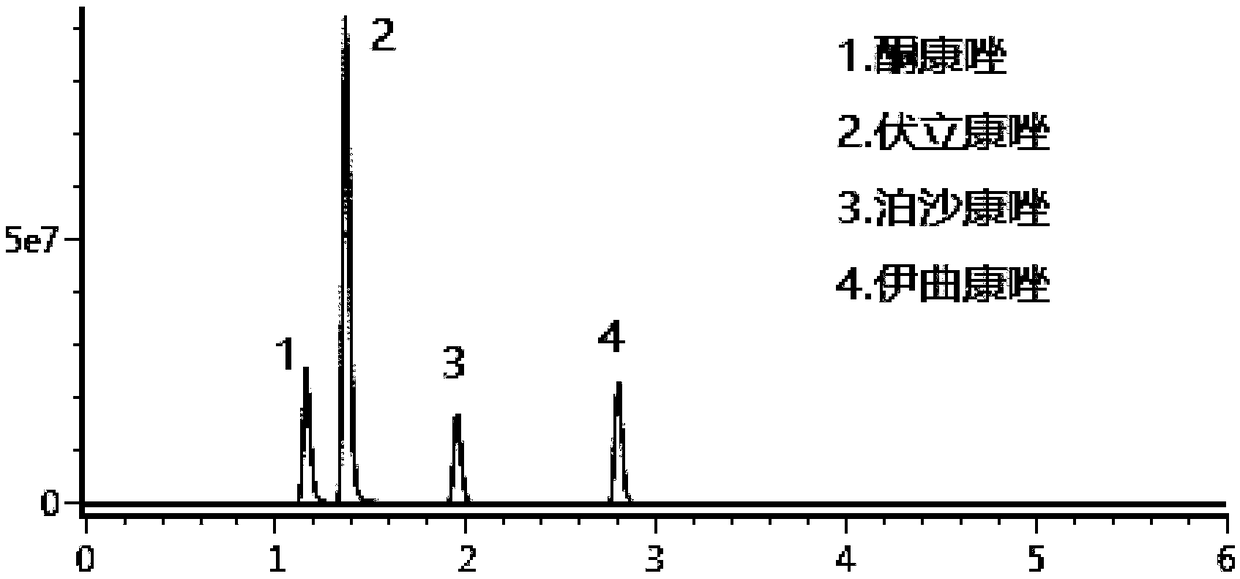
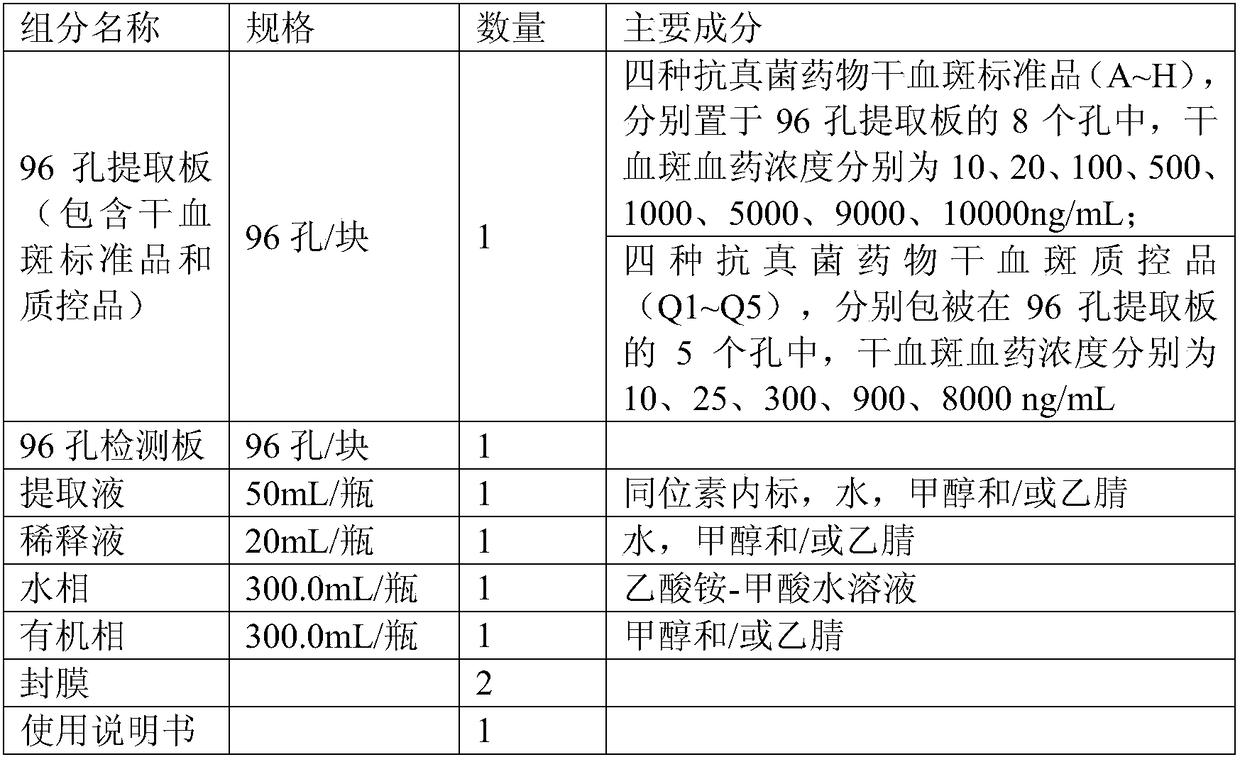
Kit for determining four antifungal drugs in dry blood spot at same time as well as application thereof
InactiveCN109030672AStrong detection specificityHigh sensitivityComponent separationAntifungal drugIsotope
The invention discloses a kit for determining four antifungal drugs in a dry blood spot at the same time, comprising extracting solution, diluent, a mobile phase, a standard substance and a quality control material; the standard substance contains ketoconazole, voriconazole, itraconazole and posaconazole; the extracting solution is composed of an isotope internal standard, water and an organic solvent; the organic solvent is methanol and / or acetonitrile; and the mobile phase comprises an organic phase and an aqueous phase, the organic phase is methanol and / or acetonitrile, and the aqueous phase is ammonium formate / acetate-formic / acetic acid aqueous solution. The invention also discloses a method for determining the four antifungal drugs in the dry blood spot at the same time by using the kit. The kit disclosed by the invention is applicable to simultaneous determination of contents of the four antifungal drugs in a dry blood spot sample, sample detection specificity is high, sensitivity is high, and stability is good.
Owner:易达精准(杭州)科技有限公司
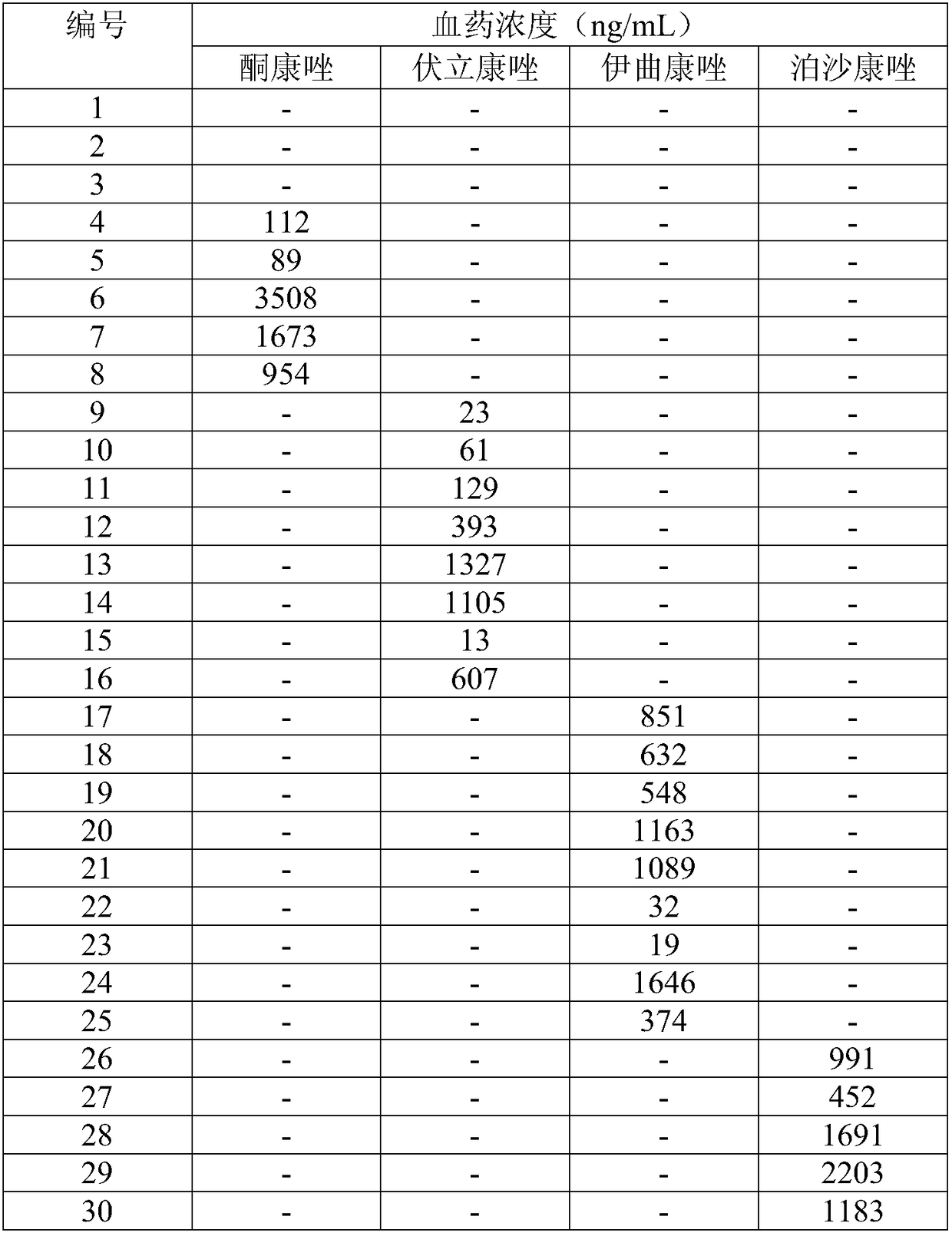
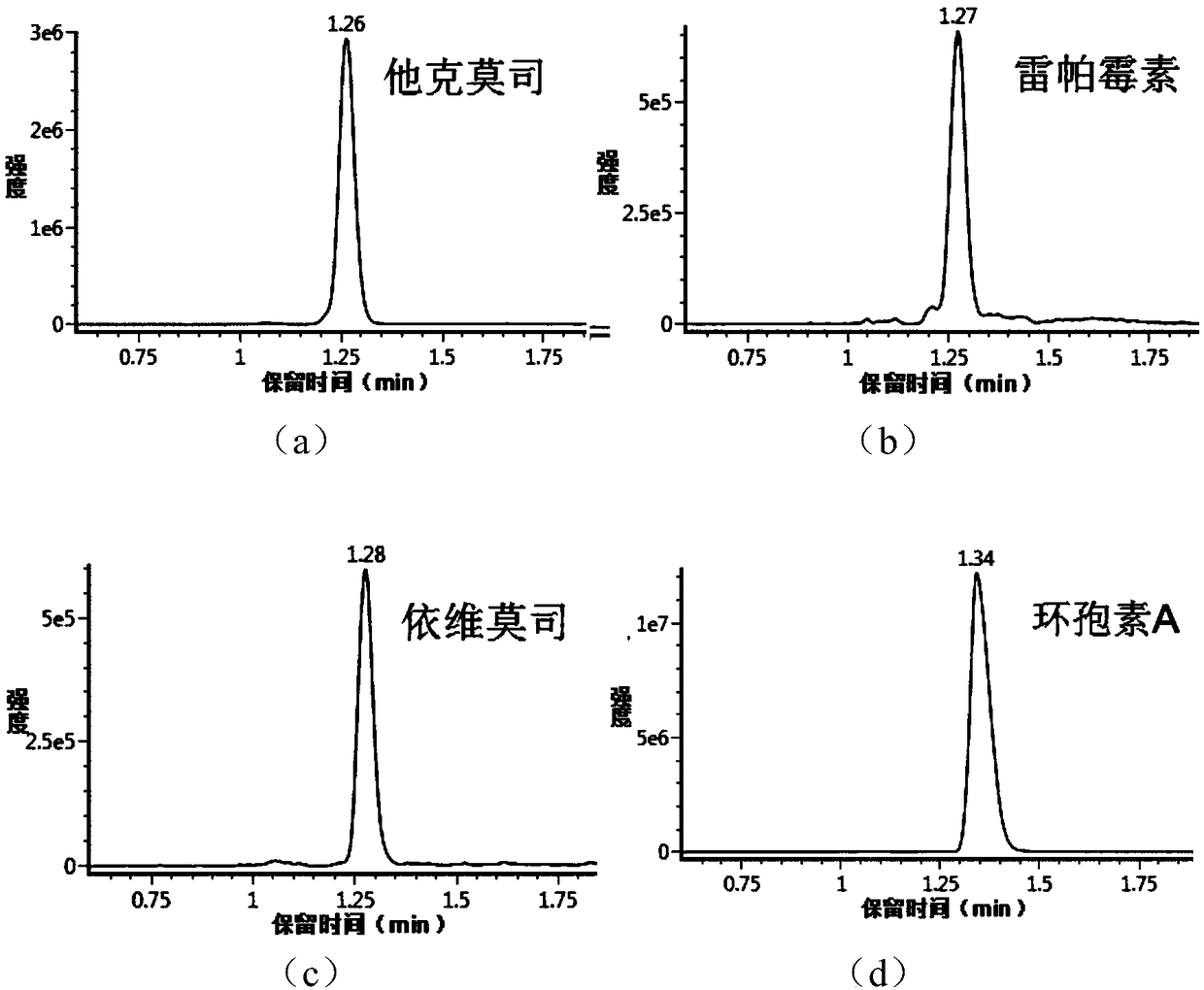
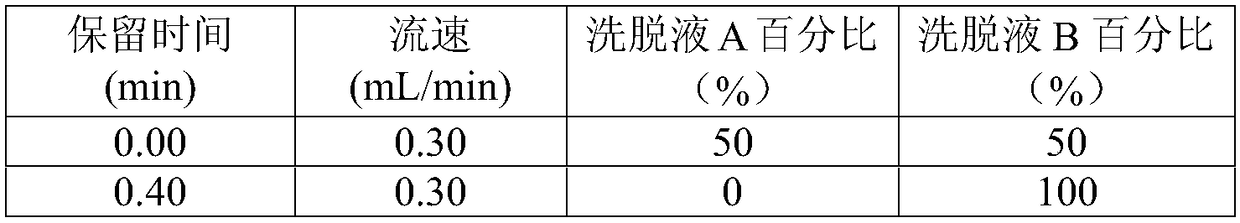
Detecting method and detecting kit for immunosuppressive agents in dried blood spots
InactiveCN109030673AApplicable common detectionEfficient detectionComponent separationEverolimusLinear regression
The invention discloses a detecting method for immunosuppressive agents in dried blood spots, and further discloses a detecting kit for the immunosuppressive agents in the dried blood spots. The detecting method comprises the steps that the standard curve dried blood spots and the to-be-detected dried blood spots are mixed with an extracting solution and an internal standard solution correspondingly and then extracted, centrifuging is conducted, and supernate is taken; the supernate is blown dry and then redissolved through a redissolving solution, and detecting is conducted through a tandem mass spectrum method; according to the peak exceeding area specific value of standard samples to an internal standard object and the concentration of the standard samples, linear regression is conducted to obtain a standard curve equation of the immunosuppressive agents; the peak exceeding area specific value of to-be-detected samples to the internal standard object is substituted into the standardcurve equation, and the content of the immunosuppressive agents in the to-be-detected samples is calculated; the extracting solution is a water solution of methyl alcohol and / or acetonitrile; and theimmunosuppressive agents are one or more of cyclosporine A, tacrolimus, sirolimus and everolimus. The detecting method can effectively detect the four types of immunosuppressive agents simultaneously, and thus the detecting efficiency is improved.
Owner:易达精准(杭州)科技有限公司
Amino acid and acyl carnitine dry blood spot control material and preparing method thereof
InactiveCN107144453AGain stabilityGain uniformityPreparing sample for investigationRed blood cellQuality control
The invention discloses an amino acid and acyl carnitine dry blood spot control material and a preparing method thereof. The dry blood spot control material is prepared from eight kinds of amino acid standard substances, 15 kinds of acyl carnitine standard substances and erythrocytes. According to the amino acid and acyl carnitine dry blood spot control material, the use quantity of standard substances is greatly reduced, the stability and homogeneity meet the requirement, and the amino acid and acyl carnitine dry blood spot control material can be stored for 4 months or longer at minus 20 DEG C. The preparing method is low in cost and easy to implement, the amino acid and acyl carnitine dry blood spot control material can be prepared in a large amount and is long in usage cycle, quality-control batch numbers do not need to be changed frequently, daily quality-control analysis is convenient, and thus the demand for the control material in clinical and daily detection is met.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
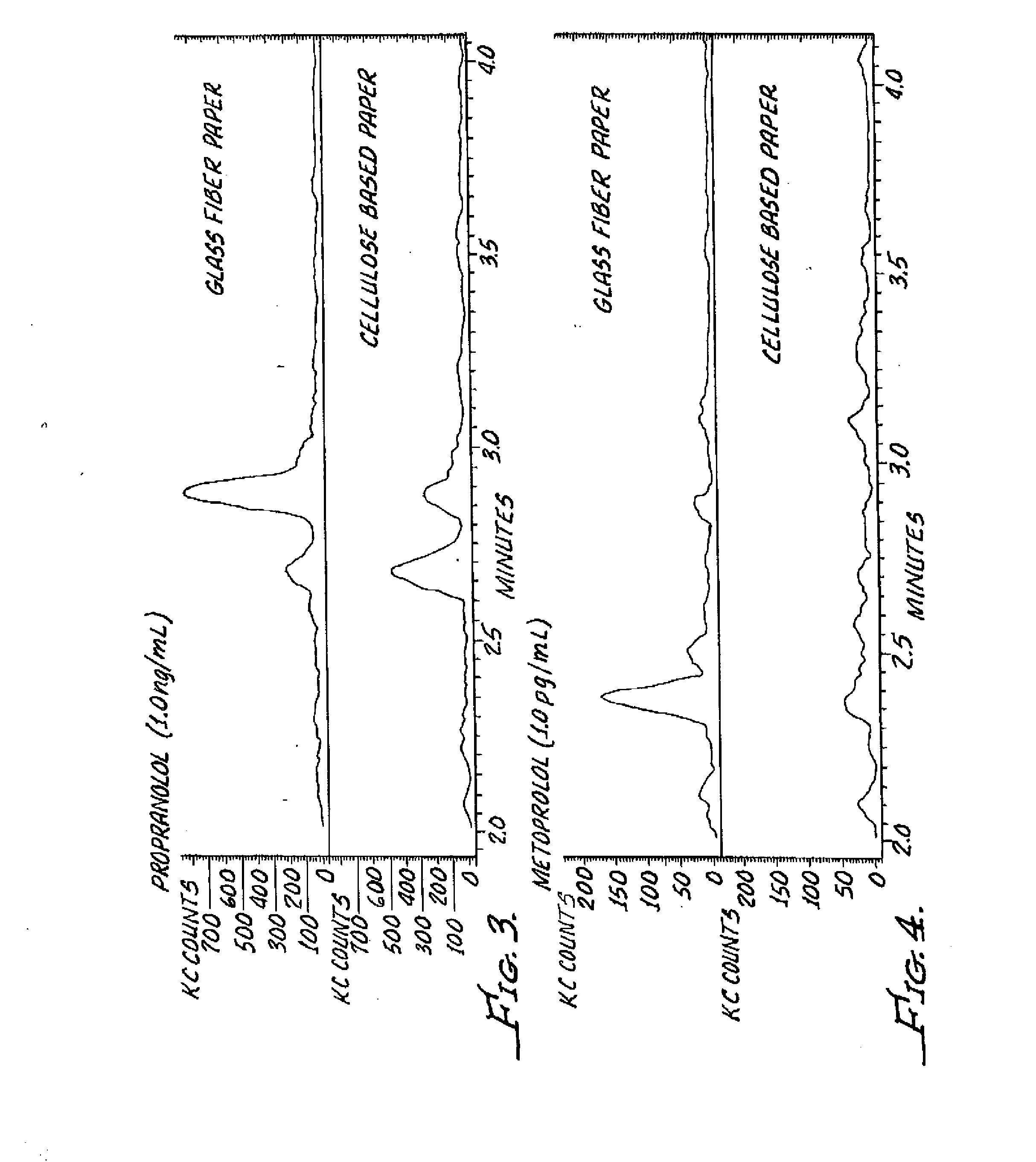
Dried blood spotting paper device and method
InactiveUS9110053B2Preparing sample for investigationLaboratory glasswaresGlass fiberMass Spectrometry-Mass Spectrometry
A dried blood-spotting device includes a carrier card having a window therethrough along with a punchable glass fiber paper disposed in the window for a uniform absorption of a blood droplet sample into a homogeneous circular sport. The glass fiber paper is configured for enabling improved analysis for small molecules by liquid chromatography / mass spectrometry of punched specimens of the sample absorbed glass fiber paper.
Owner:AGILENT TECH INC
Beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
The invention discloses a beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) applicable for early-pregnancy and mid-pregnancy prenatal screening and a preparation method thereof. The kit comprises the following main components of an experimental buffer solution, a concentrated washing solution, an enhancement solution, a reaction plate, a standard product and a quality control product of filter paper dried blood spots and a europium marker. The method for preparing the kit according to the invention comprises the following steps of: 1. preparing the experimental buffer solution, the concentrated washing solution and the enhancement solution; 2. coating the reaction plate; 3. preparing the standard product and the quality control product; 4. preparing the europium marker; 5. separately loading; 6. sticking labels; and 7. assembling into a finished product. The invention has the characteristics that the accuracy and the sensitivity of a detected result are high, the stability is good, and a detecting method is economical, convenient and safe and has no traumatic property and the like. A filter paper dried blood spot technology is applied to prenatal screening work, which is beneficial to blood specimen collection, storage and transportation and ensures the experimental reliability.
Owner:广州市丰华生物股份有限公司
Polystyrene magnetic bead based dried blood spot genome extraction kit and extraction method
InactiveCN109402113AImprove uniformitySmall particle sizeMicrobiological testing/measurementDNA preparationMagnetic beadPolystyrene
The invention relates to a polystyrene magnetic bead based dried blood spot genome DNA extraction kit. The kit comprises polystyrene magnetic beads, splitting liquid, combination liquid, washing liquid and nucleic acid eluting liquid. The polystyrene magnetic beads are polystyrene coated gamma-iron trioxide hydroxyl magnetic beads. The invention further provides a dried blood spot genome DNA extraction method in which the kit is adopted for extraction. The method includes steps: S1, splitting dried blood spots by protease K and the splitting liquid, and taking supernatant to obtain a splittingproduct; S2, adding the polystyrene magnetic beads and the combination liquid into the splitting product, well mixing, transferring to a magnetic frame, and discarding solution; S3, adopting the washing liquid for washing; S4, adopting the eluting liquid for DNA eluting from the magnetic beads with DNA, so that genome DNA is obtained.
Owner:GUANGDONG ASCENDAS GENOMICS TECH CO LTD
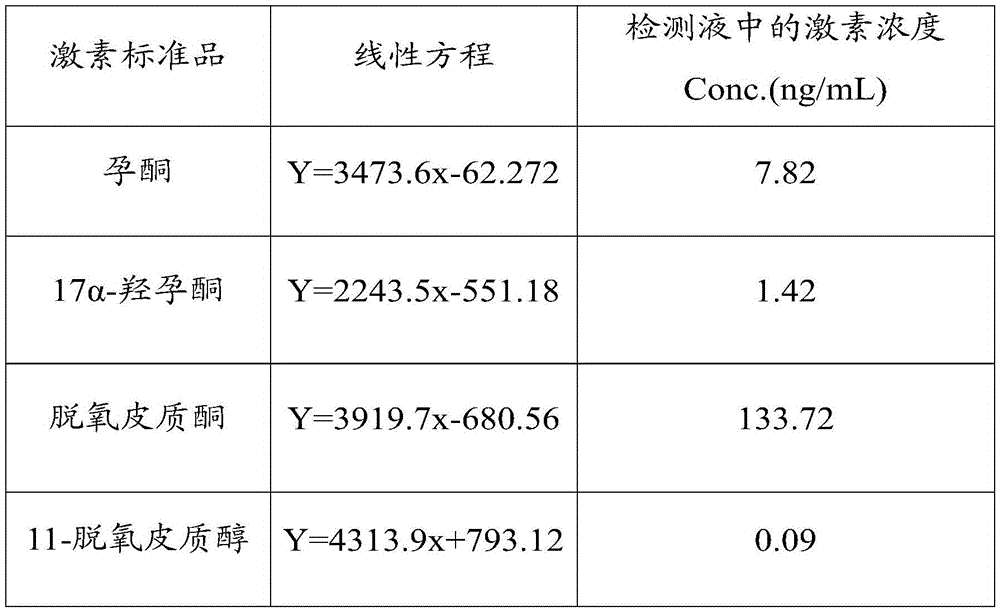
Method for detecting steroid hormone in blood
ActiveCN113933412AImprove stabilityGood reproducibilityComponent separationRed blood cellPregnanetriolone
The invention relates to a method for detecting steroid hormones in blood. The method comprises the following steps: preparation of a standard curve dried blood spot sample: preparing red blood cells by using whole blood; by taking hormone-removed serum as a blank matrix, adding standard substances such as 17alpha-hydroxyprogesterone, androstenedione, cortisol, 21-deoxycortisol and 11-deoxycortisol solutions to prepare a series of serum standard curve working solutions; mixing the red blood cells with the serum standard curve working solution, and preparing a standard curve dried blood spot sample by using the obtained mixed solution; pretreatment of the dried blood spot sample: taking the dried blood spot sample, adding an internal standard-containing extraction liquid, extracting, centrifuging, drying, adding a reconstitution fluid, centrifuging, and determining by adopting liquid chromatography-tandem mass spectrometry. The detection method disclosed by the invention is simple in pretreatment operation, short in time consumption, high in stability and good in reproducibility, and various steroid isomers can be efficiently separated at the same time.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
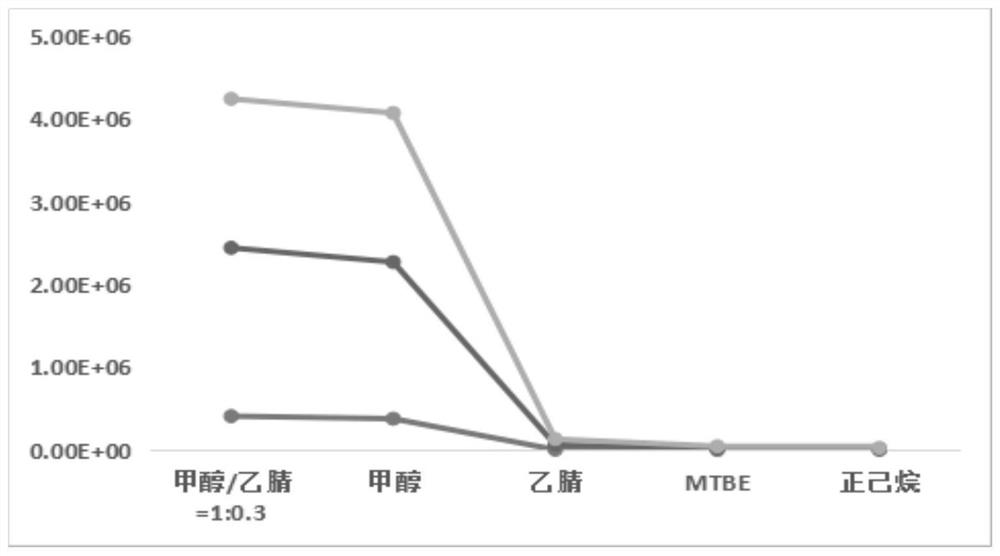
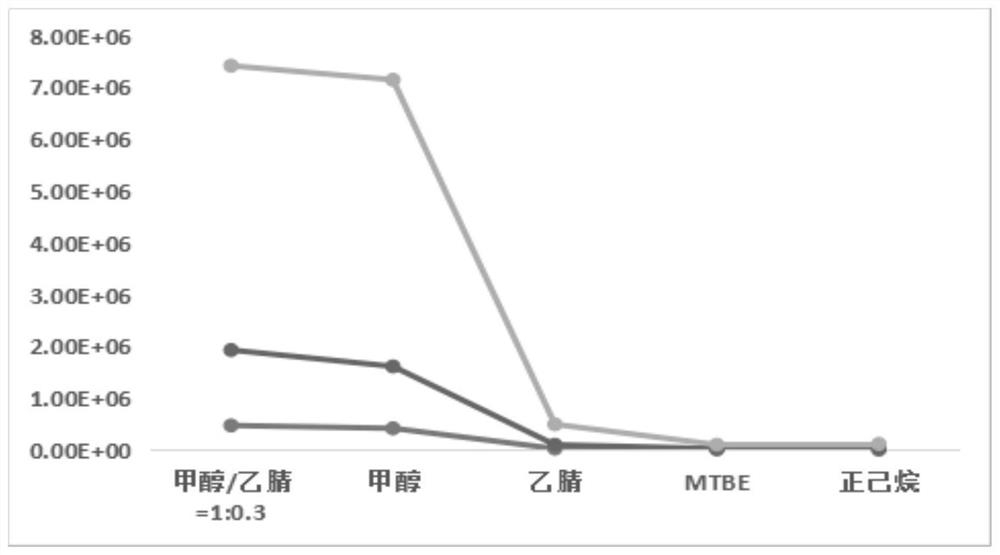
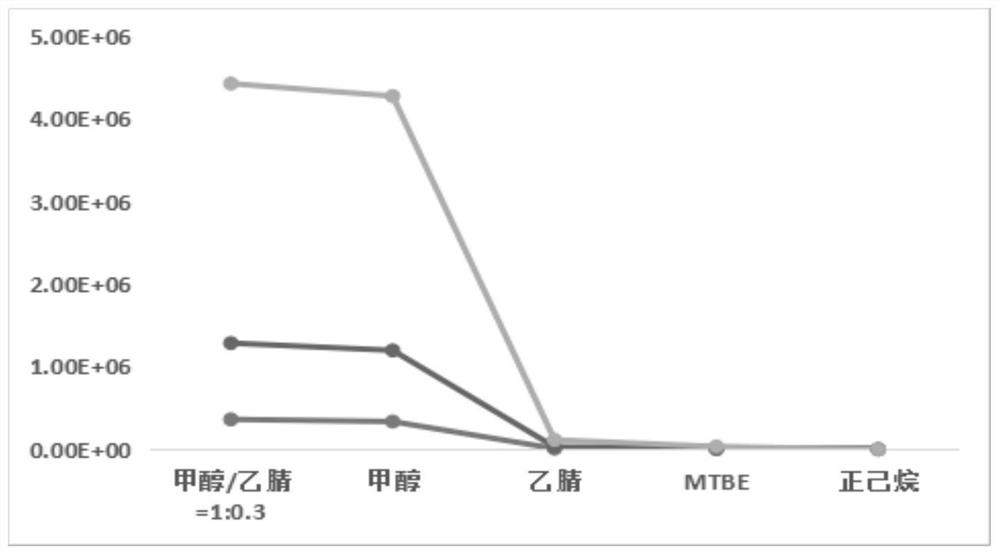
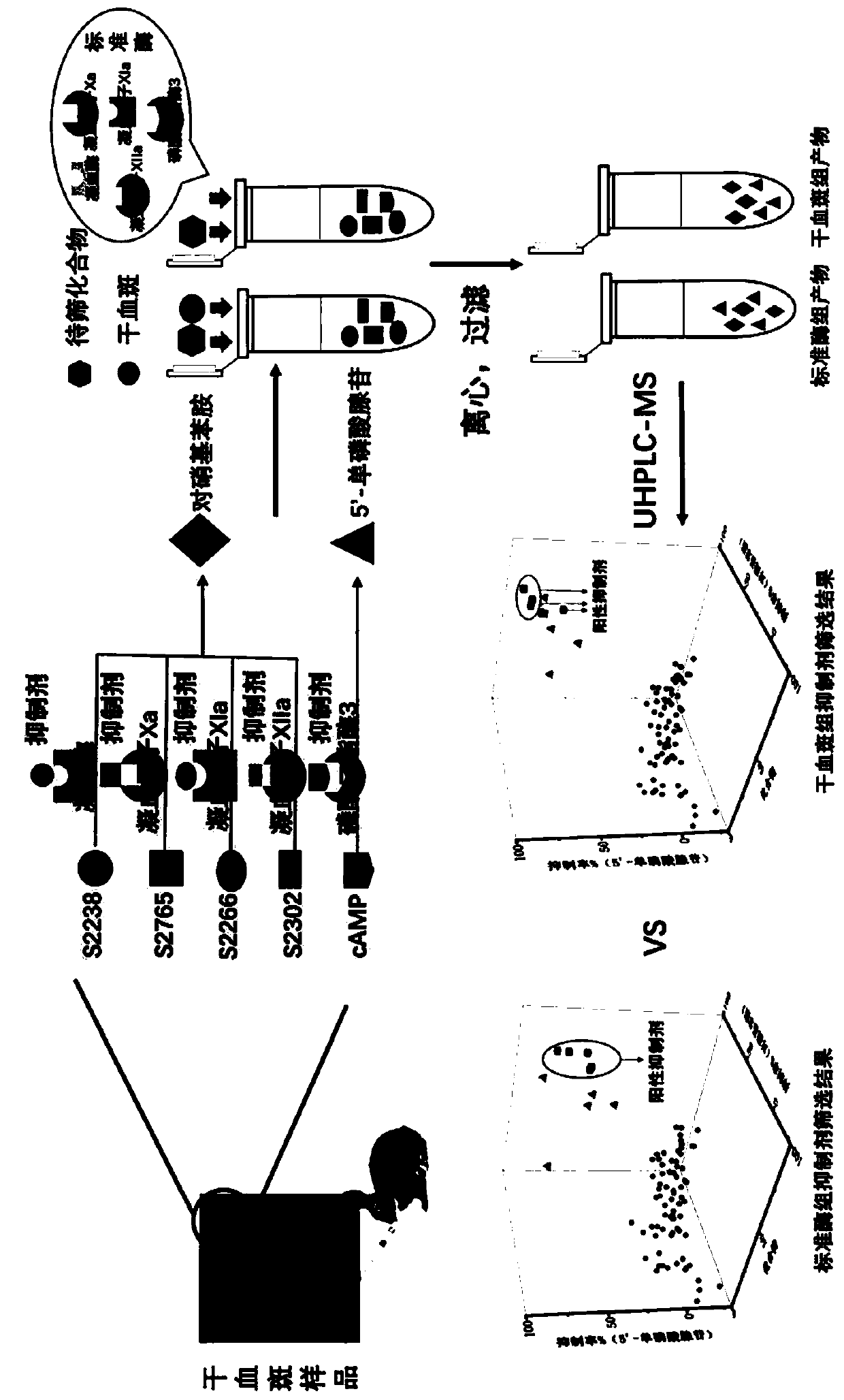
Liquid chromatography-mass spectrometry screening method for multi-target antithrombotic active substance
ActiveCN110568117AReduce economic costsHigh detection sensitivityComponent separationScreening methodMass spectrometric
The invention discloses a liquid chromatography-mass spectrometry screening method for a multi-target antithrombotic substance based on a dried blood spot sample, and belongs to the field of drug screening. The method comprises the following steps of preparing a rehydrated dry blood spot sample, optionally selecting a plurality of substrates of key target enzymes involved in thrombosis processes,mixing a plurality of substrate standard solutions with a substance to be screened, adding a mixture into the rehydrated dry blood spot sample, treating a reaction solution, and carrying out liquid chromatography-mass spectrometry analysis to screen to obtain a substance with multi-target antithrombotic activity. The screening method of the invention has the advantages of simplicity and rapidness,high accuracy, high specificity, a large number of samples can be continuously analyzed, the antithrombotic substance capable of inhibiting one or more target enzymes at the same time can be screenedout at the same time according to the screening method, action targets of the screened active substance are clear, and a theoretical basis is laid for subsequent drug development. Reagents are easy to obtain, the operation automation degree is high, clinical popularization and promotion are easy, and the commercial value is high.
Owner:MARINE BIOMEDICAL RES INST OF QINGDAO CO LTD
Liquid chromatography-tandem mass spectrometry detection method for plurality of lipid-soluble vitamins in dried blood spots
InactiveCN110174476AThe detection method is simple and sensitiveStrong specificityComponent separationRetention timeNitrogen gas
The invention discloses a liquid chromatography-tandem mass spectrometry detection method for a plurality of lipid-soluble vitamins in dried blood spots. The method comprises the following steps: preparing 1 to 3 blood stain samples, placing the 1 to 3 blood stain samples in a centrifugal tube, adding first extraction liquid, and performing vortex treatment for 25 to 35 minutes; adding an internalstandard solution and second extraction liquid, and performing vortex treatment for 2 to 4 minutes; centrifuging the mixture, transferring a supernatant into another centrifugal tube, performing blowdrying with nitrogen gas, adding reconstitution fluid, performing vortex treatment for 2 to 3 minutes, and sampling the reconstitution fluid for analysis; injecting a dry blood spot sample solution prepared in the step (1) into a LC-MS / MS (Liquid Chromatograph-Tandem Mass Spectrometer), and recording a chromatogram; and acquiring the retention time and mass spectrum data of the plurality of lipid-soluble vitamins. The detection method has the advantages of easiness, sensitivity, rapidness, accuracy, high specificity and high repeatability, and can be used for detecting the plurality of lipid-soluble vitamins in the dried blood spots.
Owner:合肥谱佳医学检验实验室有限公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com