Newborn total galactose detection kit, as well as application method and preparation method thereof
A detection kit and galactose technology, applied in the screening field of neonatal galactosemia, can solve the problems of high work intensity, poor screening accuracy of neonatal galactosemia, slow detection speed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Neonatal total galactose detection kit
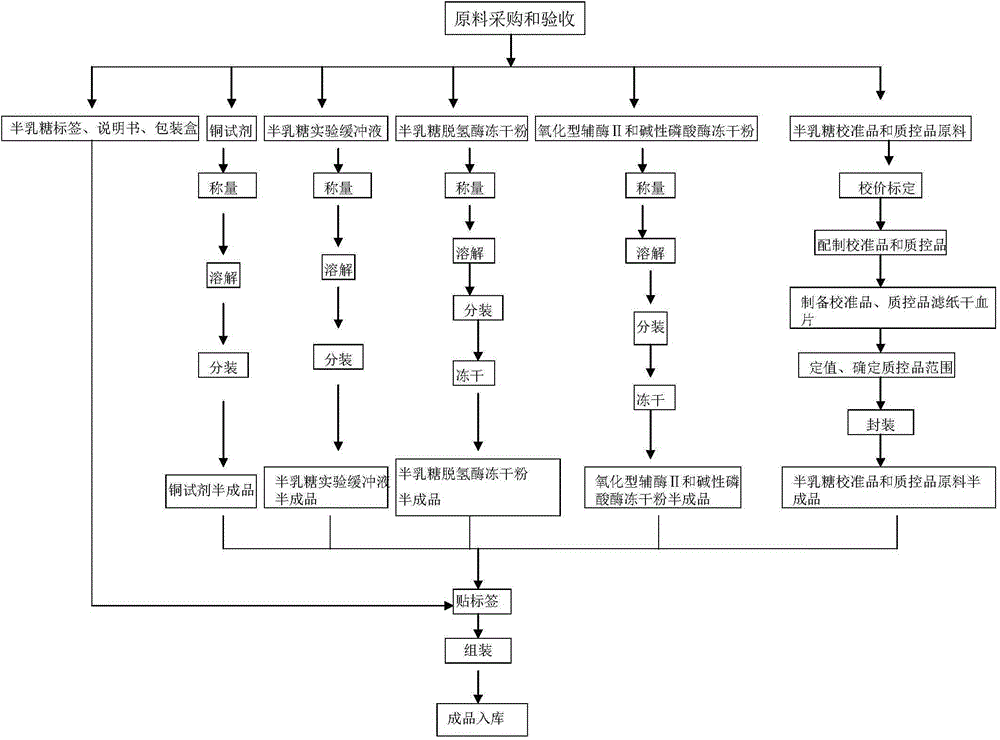
[0059] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
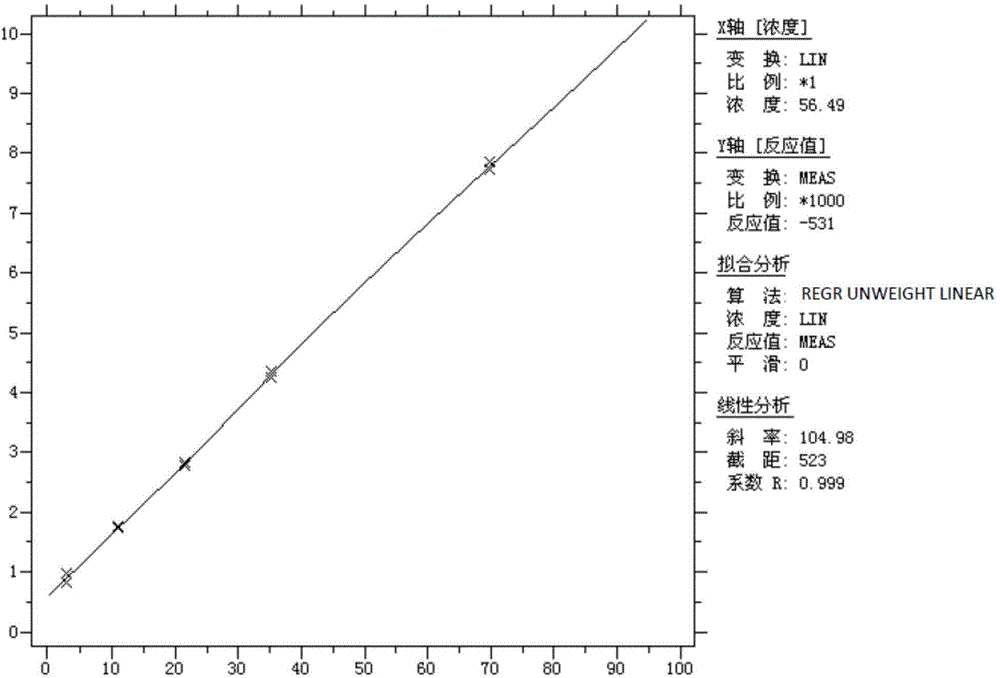
[0060] Wherein, the experimental buffer is a Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 10 mmol / L, the molar concentration of KCl is 50 mmol / L, and the pH is 8.0.
[0061]The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, which includes alkaline phosphatase with a concentration of 1000U / mL, and the concentration is 46mg / mL oxidized coenzyme II, Tris-HCl with a molar concentration of 1...
Embodiment 2
[0088] Neonatal total galactose detection kit
[0089] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
[0090] Wherein, the experimental buffer is Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 10 mmol / L, and the molar concentration of KCl is 10 mmol / L.
[0091] The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is as follows: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, wherein the concentration of alkaline phosphatase is 25U / mL, and the concentration of oxidized coenzyme II The concentration of coenzyme II is 1000mg / mL, the molar concen...
Embodiment 3
[0097] Neonatal total galactose detection kit
[0098] Neonatal total galactose detection kit contains the following components: 1) Experimental buffer; 2) Mixed lyophilized powder of alkaline phosphatase and oxidized coenzyme II; 3) Lyophilized powder of galactose dehydrogenase; 4) Galactose Calibrator and quality control of filter paper dry blood film; 5) Copper reagent; 6) 96-well microwell blank white reaction plate.
[0099] Wherein, the experimental buffer is a Tris-HCl buffer containing KCl, wherein the molar concentration of Tris-HCl is 100 mmol / L, and the molar concentration of KCl is 100 mmol / L.
[0100] The preparation method of the mixed freeze-dried powder of alkaline phosphatase and oxidized coenzyme II is as follows: a) prepare the mixed solution of alkaline phosphatase and oxidized coenzyme II, wherein the concentration of alkaline phosphatase is 5000U / mL, and the concentration of oxidized coenzyme II The concentration of coenzyme II is 10mg / mL, the molar co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com